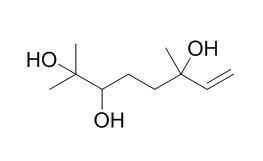
2,6-Dimethyl-7-octene-2,3,6-triol
Reference standards.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Natural Product Sciences2024, 30(4):254-261.
Molecules.2019, 24(24),4583
Chemistry of Plant Materials.2016, 33-46
Sci Rep. 2017, 12953(7)
Int J Mol Sci.2019, 20(21):E5488
Korean J. Food Preserv.2023, 30(4):663-668.
Int J Mol Sci.2023, 24(8):7045.
Front Microbiol.2023, 14:1232039.
Food Chem.2017, 228:301-314
Nutrients2020, 12(2):488
Related and Featured Products
ACS Symposium series. American Chemical Society,1986, (30):85-98.
Precursors of Papaya (Carica papaya, L.) Fruit Volatiles.[Reference:
WebLink]
METHODS AND RESULTS:
Oxygenated terpenoids derived from linalool, a major constituent among papaya (C.papaya, L.) fruit volatiles, were studied by capillary gas chromatography (HRGC) and combined capillary gas chromatography-mass spectrometry (HRGC-MS). Using a sample preparation technique suitable for the separation and enrichment of polar compounds, the two diastereoisomers of 6,7-epoxy-linalool, 2,6-dimethyl-1,7-octadiene-3,6-diol, 2,6-dimethyl-3,7-octadiene-2,6-diol, (E)- and (Z)-2,6-dimethyl-2,7-octadiene-l,6-diol, and 2,6-Dimethyl-7-octene-2,3,6-triol were identified for the first time.
CONCLUSIONS:
Each of four diastereoisomeric epoxy-linalool oxides in their furanoid and pyranoid forms were also detected for the first time as natural plant constituents. Biogenetic pathways of formation and metabolization of the oxygenated linalool derivatives are discussed.