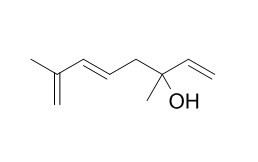
Hotrienol
Hotrienol is an excellent fruity smelling compound, could be a flavouring ingredient. Hotrienol has antioxidant capacity. Hotrienol and methyl syringate can be considered non-specific chemical markers of S. hortensis honey.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Nat Prod Communications2018, 10.1177
ARPN Journal of Eng.& Applied Sci.2016, 2199-2204
Molecules2022, 27(9):2613.
Nat Commun.2025, 16(1):4121.
Foods.2024, 13(11):1739.
Pest Manag Sci.2019, 75(9):2530-2541
Food Chem.2022, 378:131975.
Molecules.2021, 26(3):695.
Bioorg Med Chem.2020, 28(12):115553.
PLoS One.2022, 17(6):e0268505.
Related and Featured Products
Chem Biodivers. 2015 Jul;12(7):1047-56.
Antioxidant capacity and chemical profiles of Satureja montana L. Honey: hotrienol and syringyl derivatives as biomarkers.[Pubmed:
26172325 ]
The present study is focused on the antioxidant capacity and chemical profiling of eight Croatian Satureja montana L. honey samples.
METHODS AND RESULTS:
Among the 20 compounds obtained by headspace solid-phase microextraction (HS-SPME) and identified by GC-FID and GC/MS analyses, Hotrienol was predominant (75.9-81.7%). The honey matrix volatile/semivolatile profile was investigated by ultrasonic solvent extraction (USE) followed by GC-FID and GC/MS analyses. The major compounds identified by this latter method were the sinapic-acid derivatives methyl syringate (36.2-72.8%) and syringaldehyde (2.2-43.1%).
CONCLUSIONS:
Direct, targeted HPLC-DAD analyses of the native honey samples revealed the presence of methyl syringate (7.10-39.60 mg/kg) and syringic acid (0.10-1.70 mg/kg). In addition, the total phenolic content of the samples was determined by the Folin Ciocalteu assay (311.0-465.9 mg GAE/kg), and the antioxidant capacity was evaluated by the DPPH radical-scavenging activity (0.5-1.0 mmol TEAC/kg) and the ferric reducing antioxidant power (2.5-5.1 mmol Fe(2+) /kg).
J Agric Food Chem. 2003 Jul 2;51(14):4036-9.
A practical and convenient synthesis of hotrienol, an excellent fruity smelling compound.[Pubmed:
12822943]
METHODS AND RESULTS:
The practical and convenient synthesis of Hotrienol, which is an excellent fruity smelling compound, has been performed by the ene-type chlorination of linalyl acetate and then dehydrochlorinated by lithium bromide and lithium carbonate in DMF, followed by hydrolysis in three steps with an overall yield of 55%.
Food Chem. 2015 Jan 15;167:290-8.
Fractionation and identification of minor and aroma-active constituents in Kangra orthodox black tea.[Pubmed:
25148991]
The aroma constituents of Kangra orthodox black tea were isolated by simultaneous distillation extraction (SDE), supercritical fluid extraction and beverage method.
METHODS AND RESULTS:
The aroma-active compounds were identified using gas chromatography-olfactometry-mass spectrometry. Geraniol, linalool, (Z/E)-linalool oxides, (E)-2-hexenal, phytol, β-ionone, Hotrienol, methylpyrazine and methyl salicylate were major volatile constituents in all the extracts. Minor volatile compounds in all the extracts were 2-ethyl-5-methylpyrazine, ethylpyrazine, 2-6,10,14-trimethyl-2-pentadecanone, acetylfuran, hexanoic acid, dihydroactinidiolide and (E/Z)-2,6-nonadienal. The concentrated SDE extract was fractionated into acidic, basic, water-soluble and neutral fractions. The neutral fraction was further chromatographed on a packed silica gel column eluted with pentane and diethyl ether to separate minor compounds. The aroma-active compounds identified using gas chromatography-olfactometry-mass spectrometry were 2-amylfuran, (E/Z)-2,6-nonadienal, 1-pentanol, epoxylinalool, (Z)-jasmone, 2-acetylpyrrole, farnesyl acetone, geranyl acetone, cadinol, cubenol and dihydroactinidiolide.
CONCLUSIONS:
AEDA studies showed 2-hexenal, 3-hexenol, ethylpyrazine, (Z/E)-linalool oxides, linalool, (E/Z)-2,6-nonadienal, geraniol, phenylethanol, β-ionone, Hotrienol and dihydroactinidiolide to be odour active components.