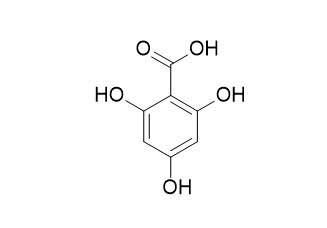
2,4,6-Trihydroxybenzoic acid
2,4,6-Trihydroxybenzoic acid may mediate its effects through a CDK- and SLC5A8-dependent pathway contributing to the prevention of colorectal cancer.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
J Pharm Biomed Anal.2018, 151:32-41
Anticancer Res.2024, 44(3):1033-1044.
J Chromatogr B Analyt Technol Biomed Life Sci.2019, 1124:323-330
Food Science and Biotechnology2022, 10.1007.
Nat Commun.2021, 12(1):681.
Sci Rep.2025, 15(1):29590.
Biomed Pharmacother.2024, 179:117410.
Theranostics.2023, 13(9):3103-3116.
Food Funct.2022, doi: 10.1039
Eur J Pharmacol.2024, 963:176280.
Related and Featured Products
Cancers, 2019,11(3):427.
The Flavonoid Metabolite 2,4,6-Trihydroxybenzoic Acid Is a CDK Inhibitor and an Anti-Proliferative Agent: A Potential Role in Cancer Prevention.[Reference:
WebLink]
Flavonoids have emerged as promising compounds capable of preventing colorectal cancer (CRC) due to their anti-oxidant and anti-inflammatory properties. It is hypothesized that the metabolites of flavonoids are primarily responsible for the observed anti-cancer effects owing to the unstable nature of the parent compounds and their degradation by colonic microflora.
METHODS AND RESULTS:
In this study, we investigated the ability of one metabolite, 2,4,6-Trihydroxybenzoic acid (2,4,6-THBA) to inhibit Cyclin Dependent Kinase (CDK) activity and cancer cell proliferation. Using in vitro kinase assays, we demonstrated that 2,4,6-THBA dose-dependently inhibited CDKs 1, 2 and 4 and in silico studies identified key amino acids involved in these interactions. Interestingly, no significant CDK inhibition was observed with the structurally related compounds 3,4,5-trihydroxybenzoic acid (3,4,5-THBA) and phloroglucinol, suggesting that orientation of the functional groups and specific amino acid interactions may play a role in inhibition. We showed that cellular uptake of 2,4,6-THBA required the expression of functional SLC5A8, a monocarboxylic acid transporter. Consistent with this, in cells expressing functional SLC5A8, 2,4,6-THBA induced CDK inhibitory proteins p21Cip1 and p27Kip1 and inhibited cell proliferation.
CONCLUSIONS:
These findings, for the first time, suggest that the flavonoid metabolite 2,4,6-THBA may mediate its effects through a CDK- and SLC5A8-dependent pathway contributing to the prevention of CRC.
China Journal of Chinese Materia Medica, 2001, 26(4):260-262.
Studies on chemical constitutents from Penthorum chinense Pursh.[Reference:
WebLink]
To study the chemical constituents of Penthorum chinense.
METHODS AND RESULTS:
Silica gel and macroporous resin were used as adsorbent for isolation and purification. The chemical structures were elucidated by spectral analysis and chemical methods.Three compounds were isolated from the water-extract of P. chinense. The structures were identified as 5-hydroxy-flavanone-7-O-β-D-glucoside (Ⅰ), 2,4,6-Trihydroxybenzoic acid (Ⅱ) and quercetin(Ⅲ), respectively.
CONCLUSIONS:
Compounds Ⅰ and Ⅱ were isolated from this plant for the first time.