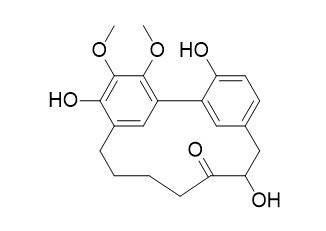
12-Hydroxymyricanone
12-Hydroxymyricanone exhibits anti-tubercular activity against Mycobacterium tuberculosis H37Rv in vitro and the MIC value of 35.8 ug/mL. It inhibits the release of nitric oxide with the IC(50) value of 30.19 muM.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Virol J.2024, 21(1):95.
Nutrients.2024, 16(15):2518.
J Formos Med Assoc.2020, S0929-6646(20)30425-3
Molecules.2019, 24(10):E1930
J Ethnopharmacol.2019, 235:406-414
Molecules.2020, 25(21):5087.
Appl. Sci.2023, 13(2), 860.
J Food Biochem.2020, 44(6):e13198.
Int J Mol Sci.2021, 22(14):7324.
J Plant Biotechnol.2023, 50:070-075.
Related and Featured Products
Phytochemistry. 2014 Jul;103:89-98.
Biological evaluation of secondary metabolites from the roots of Myrica adenophora.[Pubmed:
24810013]
METHODS AND RESULTS:
Bioassay-guided fractionation of the roots of Myrica adenophora led to isolation of 24 known compounds and hitherto unknown compounds, including three A-type proanthocyanidins [adenodimerins A-C], two esters of sucrose [myricadenins A and B ], and the phenolic glycoside 6'-O-galloyl orbicularin. Spectroscopic analyses were used to determine their structures. Adenodimerin A, myricananin C, and myricetin showed strong 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical scavenging activities, with SC50 values of 7.9, 16.3, and 15.9 μM, respectively. Adenodimerin A, myricanone, myricananin C, (-)-myricanol, myricanol 11-O-β-D-glucopyranoside, and myricetin showed stronger 2,2'-azino-bis-3-ethylbenzthiazoline-6-sulphonic acid (ABTS) radical scavenging activities than the positive control, with SC50 values of 7.5, 19.6, 12.0, 22.3, 19.6, and 15.6 μM, respectively.
CONCLUSIONS:
5-Deoxymyricanone, porson, 12-Hydroxymyricanone (-)-myricanol, and (+)-galeon exhibited anti-tubercular activity against
Mycobacterium tuberculosis H37Rv in vitro and MICs values of 25.8, 40.0, 35.8, 30.0, and 15.0 μg/mL, respectively. Myricadenin A, myricanone, myricananin C, and (-)-myricanol exhibited anti-inflammatory activities in the iNOS assay with EC50 values of 18.1, 1.00, 13.0, and 7.5 μM, respectively.
Bioorg Med Chem. 2008 Sep 15;16(18):8510-5.
Cyclic diarylheptanoids from Myrica nana inhibiting nitric oxide release.[Pubmed:
18723353 ]
METHODS AND RESULTS:
Investigation of the roots of Myrica nana afforded five new cyclic diarylheptanoids, myricananins A-E (1-5), two new artifacts of myricananins A and B (6-7), and four known compounds, 12-Hydroxymyricanone (8), alnusonol (9), myricatomentogenin (10), and actinidione (11). The structures of these new compounds were established by detailed spectroscopic methods. The stereochemistry of compounds 1 and 2 were determined by single-crystal X-ray diffraction. In exception of compounds 2, 6 and 10, all the other compounds were examined for their inhibitory effects on nitric oxide production in lipopolysaccharides-activated macrophages.
CONCLUSIONS:
Compounds 1, 3, 7, 8 and 9 inhibited the release of nitric oxide with IC(50) values of 45.32, 63.51, 52.81, 30.19 and 46.18muM, respectively. Furthermore, compound 1 was found to inhibit the expression of inducible nitric oxide synthase.