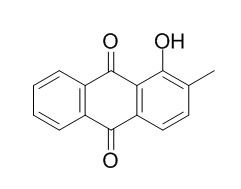
1-Hydroxy-2-methylanthraquinone
1-Hydroxy-2-methylanthraquinone exhibits promising larvicidal activity.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Kaohsiung J Med Sci.2023, 10.1002/kjm2.12764
J Pharm Biomed Anal.2017, 140:274-280
Cell Death Differ.2021, 1-8.
Applied Biological Chemistry2021, 64(4)
Eur Rev Med Pharmacol Sci.2020, 24(9):5127-5139.
Int Immunopharmacol.2020, 90:107268.
Evid Based Complement Alternat Med.2017, 2017:1583185
Int J Oncol.2019, 55(1):320-330
Processes2021, 9(1), 153;
Journal of Molecular Liquids2022, 364:120062.
Related and Featured Products
J Nat Prod. 2011 Jan 28;74(1):82-5.
Isolation, structure elucidation, and cytotoxic evaluation of furanonaphthoquinones from in vitro plantlets and cultures of Streptocarpus dunnii.[Pubmed:
21174407]
METHODS AND RESULTS:
Two new furanonaphthoquinones, (3R)-7-methoxy-α-dunnione (5) and (3R)-6-hydroxy-7-methoxy-α-dunnione (6), along with the known (3R)-dunnione (1), (3R)-α-dunnione (2), (3R)-7-hydroxy-α-dunnione (3), and 1-Hydroxy-2-methylanthraquinone (4), were isolated from in vitro cultures of Streptocarpus dunnii. The structures of compounds 5 and 6 were established by spectroscopic means. This is the first report of hydroxylated furanonaphthoquinones in a Streptocarpus species.
CONCLUSIONS:
Compounds 1-3 demonstrated cytotoxic activity against a range of breast cancer and pancreatic tumor cell lines.
Nat Prod Res. 2009;23(14):1322-9.
A new anthraquinone from Morinda citrifolia roots.[Pubmed:
19735047]
METHODS AND RESULTS:
An investigation of Morinda citrifolia roots afforded a new anthraquinone, 2-ethoxy-1-hydroxyanthraquinone (1), along with five other known anthraquinones: 1-Hydroxy-2-methylanthraquinone (2), damnacanthal (3), nordamnacanthal (4), 2-formyl-1-hydroxyanthraquinone (5) and morindone-6-methyl-ether (6). This is the first report on the isolation of morindone-6-methyl-ether (6) from this plant. The structures of these compounds were elucidated based on spectroscopic analyses such as NMR, MS and IR.
CONCLUSIONS:
Biological evaluation of five pure compounds and all the extracts against the larvae of Aedes aegypti indicated 1-Hydroxy-2-methylanthraquinone (2) and damnacanthal (3) were the extracts to exhibit promising larvicidal activities.
Chem Biodivers. 2015 Jan;12(1):148-52.
Two new hydronaphthoquinones from Sinningia aggregata (Gesneriaceae) and cytotoxic activity of aggregatin D.[Pubmed:
25641842]
METHODS AND RESULTS:
Two new hydronaphthoquinones, aggregatins E and F (1 and 2, resp.) were isolated from the tubers of Sinningia aggregata (Ker-Gawl.) Wiehler (Gesneriaceae), along with twelve known compounds aggregatin D (3), tectoquinone (4), 1-Hydroxy-2-methylanthraquinone (5), icosyl ferulate (6), pustuline (7), 1,6-dihydroxy-2-methylanthranquinone (8), 6-hydroxy-2-methylanthraquinone (9), 7-hydroxy-2-methylanthraquinone (10), tyrosol (11), halleridone (12), calceolarioside B (13), and cornoside (14). All compounds were identified by analysis of spectroscopic and spectrometric data. Compounds 3, 4, and 10 had already been reported in this species.
CONCLUSIONS:
Compounds 2 and 3 were evaluated against several tumor cell lines, but only 3 exhibited activities against UACC-62, 786-0 and OVCAR-3 cell lines, with IC50 values of 12.3, 12.8 and 0.3 μg/ml, respectively, without toxic effects on non-cancer cell line HaCat (human keratinocyte).