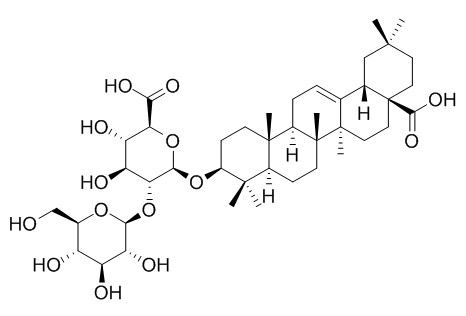
Zingibroside R1
Zingibroside R1 has anti-HIV-1 activity.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Chinese Journal of Tissue Engineering Research2024, 28(8):1149-1154.
Toxicol In Vitro.2023, 86:105521.
Front Pharmacol.2023, 14:1244655.
Eur J Ther.2023, 29(4):900-906.
Development.2024, 151(20):dev202518.
Phytomedicine.2019, 59:152785
Toxicol Appl Pharmacol.2022, 434:115815.
Molecules.2018, 23(9):E2121
Phytomedicine2022, 104:154318
J Appl Biol Chem2023, 66:455−461
Related and Featured Products
Planta Med. 1994 Jun;60(3):240-3.
Inhibitory effect of some triterpenoid saponins on glucose transport in tumor cells and its application to in vitro cytotoxic and antiviral activities.[Pubmed:
8073091]
METHODS AND RESULTS:
The effects of some triterpenoid saponins on glucose transport in Ehrlich ascites tumor (EAT) cells were examined by measuring 2-deoxy-D-glucose (2-DG) uptake. The correlation of the effects with those on the growth of a human T-cell line (MT-4) and the replication of human immunodeficiency virus in MT-4 cells was also studied.
CONCLUSIONS:
Chikusetsusaponin Ia isolated from rhizomes of Panax japonicus C. A. Meyer (Araliaceae) inhibited the 2-DG uptake (IC50 = 76.3 microM) in a competitive fashion with respect to 2-DG (Ki = 0.32 mM) and the growth of MT-4 cells with CC50 of 84.4 microM, whereas it did not show any significant anti-HIV-1 activity.
In contrast, Zingibroside R1 isolated from rhizomes of Panax zingiberensis Wu et Feng (Araliaceae) showed some anti-HIV-1 activity, which was found to be superior to that of glycyrrhizin, as well as the inhibitory effects on the 2-DG uptake by EAT cells (IC50 = 91.3 microM) and the growth of MT-4 cells (CC50 = 46.2 microM).
Chem Pharm Bull (Tokyo) . 2013;61(3):344-50.
Saponins composition of rhizomes, taproots, and lateral roots of Satsuma-ninjin (Panax japonicus)[Pubmed:
23291557]
Abstract
A new dammarane-type triterpenoid saponin, chikusetsusaponin VII (1), and nineteen known triterpenoid saponins, ginsenoside Rb1 (2), ginsenoside Rb3 (3), ginsenoside Rc (4), ginsenoside Rd (5), ginsenoside Re (6), ginsenoside Rg1 (7), ginsenoside Rg2 (8), ginsenoside Rh1 (9), notoginsenoside R1 (10), notoginsenoside R2 (11), notoginsenoside Fe (12), chikusetsusaponin IVa (13), chikusetsusaponin IV (14), chikusetsusaponin V (15), chikusetsusaponin VI (16), chikusetsusaponin FK6 (17), gypenoside XVII (18), 28-desglucosylchikusetsusaponin IV (19), and Zingibroside R1 (20), were isolated from rhizomes, taproots, and lateral roots of Panax japonicus C. A. Meyer, so-called "Satsuma-ninjin," grown in southern Miyazaki Prefecture, Japan. The structure of new chikusetsusaponin VII was elucidated on the basis of spectral and physicochemical evidence. Although the chemical composition of the rhizome was found to be similar to that of the "Chikusetsu-ninjin," the saponin composition of lateral root of "Satsuma-ninjin" was found to be close to that of lateral root of P. ginseng. The total yield of oleanolic acid saponins of the taproot was less than that of rhizome, but the total yield of dammarane-type saponins of the taproot was found to be similar to that of rhizome.
Chem Pharm Bull (Tokyo). 2013;61(3):344-50.
Saponins composition of rhizomes, taproots, and lateral roots of Satsuma-ninjin (Panax japonicus).[Pubmed:
23291557]
METHODS AND RESULTS:
A new dammarane-type triterpenoid saponin, chikusetsusaponin VII (1), and nineteen known triterpenoid saponins, ginsenoside Rb1 (2), ginsenoside Rb3 (3), ginsenoside Rc (4), ginsenoside Rd (5), ginsenoside Re (6), ginsenoside Rg1 (7), ginsenoside Rg2 (8), ginsenoside Rh1 (9), notoginsenoside R1 (10), notoginsenoside R2 (11), notoginsenoside Fe (12), chikusetsusaponin IVa (13), chikusetsusaponin IV (14), chikusetsusaponin V (15), chikusetsusaponin VI (16), chikusetsusaponin FK6 (17), gypenoside XVII (18), 28-desglucosylchikusetsusaponin IV (19), and Zingibroside R1 (20), were isolated from rhizomes, taproots, and lateral roots of Panax japonicus C. A. Meyer, so-called "Satsuma-ninjin," grown in southern Miyazaki Prefecture, Japan. The structure of new chikusetsusaponin VII was elucidated on the basis of spectral and physicochemical evidence. Although the chemical composition of the rhizome was found to be similar to that of the "Chikusetsu-ninjin," the saponin composition of lateral root of "Satsuma-ninjin" was found to be close to that of lateral root of P. ginseng.
CONCLUSIONS:
The total yield of oleanolic acid saponins of the taproot was less than that of rhizome, but the total yield of dammarane-type saponins of the taproot was found to be similar to that of rhizome.