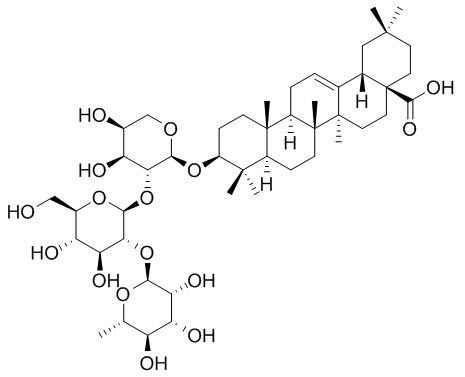
Raddeanin A
Raddeanin A, a histone deacetylases (HDACs) inhibitor, has high antiangiogenic potency, and antitumor activity, it can suppress the growth of liver and cells, it also inhibits proliferation of GC cells (BGC-823, SGC-7901 and MKN-28), induces their and inhibits the abilities of invasion, migration.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Environ Toxicol.2023, 23929.
Nutr Metab (Lond).2019, 16:31
Molecules.2019, 24(21):E3834
J. of Med. Plant Research.2013, 90-151
Pak J Pharm Sci.2019, 32(6)
Appl. Sci.2025, 15(1), 247
Pharmacol Rep.2020, 72(2):472-480.
Protoplasma.2024, 261(6):1267-1280.
J Chromatogr A.2022, 1685:463640.
Plant Commun.2024, 5(10):101005.
Related and Featured Products
Phytomedicine. 2015 Jan 15;22(1):103-10.
Raddeanin A, a triterpenoid saponin isolated from Anemone raddeana, suppresses the angiogenesis and growth of human colorectal tumor by inhibiting VEGFR2 signaling.[Pubmed:
25636878]
Raddeanin A (RA) is an active triterpenoid saponin from a traditional Chinese medicinal herb, Anemone raddeana Regel. It was previously reported that RA possessed attractive antitumor activity through inhibiting proliferation and inducing apoptosis of multiple cancer cells.
However, whether RA can inhibit angiogenesis, an essential step in cancer development, remains unknown.
METHODS AND RESULTS:
In this study, we found that RA could significantly inhibit human umbilical vein endothelial cell (HUVEC) proliferation, motility, migration, and tube formation. RA also dramatically reduced angiogenesis in chick embryo chorioallantoic membrane (CAM), restrained the trunk angiogenesis in zebrafish, and suppressed angiogenesis and growth of human HCT-15 colorectal cancer xenograft in mice. Western blot assay showed that RA suppressed VEGF-induced phosphorylation of VEGFR2 and its downstream protein kinases including PLCγ1, JAK2, FAK, Src, and Akt. Molecular docking simulation indicated that RA formed hydrogen bonds and hydrophobic interactions within the ATP binding pocket of VEGFR2 kinase domain.
CONCLUSIONS:
Our study firstly provides the evidence that RA has high antiangiogenic potency and explores its molecular basis, demonstrating that RA is a potential agent or lead candidate for antiangiogenic cancer therapy.
Biochem Biophys Res Commun. 2013 Sep 20;439(2):196-202.
Raddeanin A induces human gastric cancer cells apoptosis and inhibits their invasion in vitro.[Pubmed:
23988447]
Raddeanin A is one of the triterpenoid saponins in herbal medicine Anemone raddeana Regel which was reported to suppress the growth of liver and lung cancer cells. However, little was known about its effect on gastric cancer (GC) cells.
This study aimed to investigate its inhibitory effect on three kinds of different differentiation stage GC cells (BGC-823, SGC-7901 and MKN-28) in vitro and the possible mechanisms.
METHODS AND RESULTS:
Proliferation assay and flow cytometry demonstrated Raddeanin A's dose-dependent inhibitory effect and determined its induction of cells apoptosis, respectively. Transwell assay, wounding heal assay and cell matrix adhesion assay showed that Raddeanin A significantly inhibited the abilities of the invasion, migration and adhesion of the BGC-823 cells. Moreover, quantitative real time PCR and Western blot analysis found that Raddeanin A increased Bax expression while reduced Bcl-2, Bcl-xL and Survivin expressions and significantly activated caspase-3, caspase-8, caspase-9 and poly-ADP ribose polymerase (PARP). Besides, Raddeanin A could also up-regulate the expression of reversion inducing cysteine rich protein with Kazal motifs (RECK), E-cadherin (E-cad) and down-regulate the expression of matrix metalloproteinases-2 (MMP-2), MMP-9, MMP-14 and Rhoc.
CONCLUSIONS:
In conclusion, Raddeanin A inhibits proliferation of human GC cells, induces their apoptosis and inhibits the abilities of invasion, migration and adhesion, exhibiting potential to become antitumor drug.
Chem Pharm Bull (Tokyo). 2014;62(8):779-85.
Synthesis and biological evaluation of Raddeanin A, a triterpene saponin isolated from Anemone raddeana.[Pubmed:
25087630]
First, Raddeanin A, a cytotoxic oleanane-type triterpenoid saponin isolated from Anemone raddeana REGEL, was synthesized. Stepwise glycosylation was adopted in the synthesis from oleanolic acid, employing arabinosyl, glucosyl and rhamnosyl trichloroacetimidate as donors.
METHODS AND RESULTS:
The chemical structure of Raddeanin A was confirmed by means of (1)H-NMR, (13)C-NMR, IR, MS and elemental analysis, which elucidated the structure to be 3-O-α-L-rhamnopyranosyl-(1→2)-β-D-glucopyranosyl-(1→2)-α-L-arabinopyranoside oleanolic acid.
CONCLUSIONS:
Biological activity tests showed that in the range of low concentrations, Raddeanin A displayed moderate inhibitory activity against histone deacetylases (HDACs), indicating that the HDACs' inhibitory activity of Raddeanin A may contribute to its cytotoxicity.
J Chromatogr B Analyt Technol Biomed Life Sci. 2013 Apr 1;923-924:43-7.
Determination of Raddeanin A in rat plasma by liquid chromatography-tandem mass spectrometry: application to a pharmacokinetic study.[Pubmed:
23455073]
A simple, rapid and sensitive LC-MS/MS analysis method was developed and validated for the determination of Raddeanin A (RA) in rat plasma. Protein precipitation with three volumes of methanol as the precipitation reagent was used as the sample preparation method.
METHODS AND RESULTS:
The analysis process was performed on a Thermo Syncronis C18 column with the mobile phase of methanol-water (containing 5mM ammonium formate, pH 2.2) (85:15, v/v). RA and glycyrrhetinic acid (internal standard) were monitored under negative electrospray ionization in multiple reaction monitoring (MRM) mode. Retention time of RA and IS were 2.1 min and 3.5 min, respectively. The limit of detection was 5 ng/mL and the linear range was 50-50,000 ng/mL. The intra-day and inter-day precision was 1.87-2.94% and 3.25-5.36%, and the intra-day and inter-day accuracy ranged from 5.9% to 10.5% and 5.6% to 11.1%, respectively. The absolute recovery was above 90.3%.
CONCLUSIONS:
The method has been successfully translated to the pharmacokinetic study of RA in rats after intravenous and intraperitoneal administration (0.75 mg/kg).