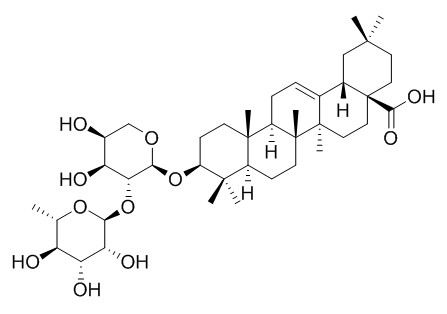
Beta-Hederin
Beta-Hederin has antileishmanial activity; it has apoptotic effect on breast cancer cells, it could be a promising candidate for chemotherapy of breast cancer.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Cytotechnology.2017, 69(5):765-773
Front Pharmacol.2018, 9:236
Inflammation.2020, 43(5):1716-1728.
Prev Nutr Food Sci.2024, 29(4):504-511.
Cell Commun Signal.2024, 22(1):597.
Food Funct.2022, D1FO03838A.
J Biotechnol.2020, 318:10-19.
J Microbiol Biotechnol.2020, 30(2):178-186.
J Lipid Res.2024, 65(10):100640.
J Chromatogr Sci.2020, 58(6):485-493.
Related and Featured Products
Pulm Pharmacol Ther. 2015 Apr;31:92-8.
A systematic study on the influence of the main ingredients of an ivy leaves dry extract on the β2-adrenergic responsiveness of human airway smooth muscle cells.[Pubmed:
25234924]
The bronchospasmolytic and secretolytic effects of ivy leaves dry extracts can be explained by an increased β2-adrenergic responsiveness of the bronchi. Recently, it was shown that α-hederin inhibits the internalization of β2-adrenergic receptors (ß2AR) under stimulating conditions. α-Hederin pretreated alveolar type II cells and human airway smooth muscle cells revealed an increased ß2AR binding and an elevated intracellular cAMP level, respectively.
METHODS AND RESULTS:
In order to identify whether additional compounds also mediate an increased β2-adrenergic responsiveness, we examined the ingredients of an ivy leaves dry extract (EA 575) protocatechuic acid, neochlorogenic acid, chlorogenic acid, cryptochlorogenic acid, rutin, kaempferol-3-O-rutinoside, 3,4-, 3,5- and 4,5-dicaffeoylquinic acid, hederacoside B, and Beta-Hederin. Within all the tested substances, only β-hederin inhibited the internalization of GFP-tagged ß2AR in stably transfected HEK293 cells. Using fluorescence correlation spectroscopy Beta-Hederin (1 μM, 24 h) pretreated HASM cells showed a statistically significant increase in the ß2AR binding from 33.0 ± 8.9% to 44.1 ± 11.5% which was distributed with 36.0 ± 9.5% for τbound1 and 8.1 ± 2.6% for τbound2, respectively (n = 8, p < 0.05). The increased binding was selectively found for the receptor-ligand complex with unrestricted lateral mobility (τbound1 of 0.9 ± 0.1 ms, D1 = 9.1 ± 0.2 μm(2)/s, n = 8), whereas the binding of ß2AR with hindered lateral mobility (τbound2 of 64.2 ± 47.6 ms, D2 = 0.15 ± 0.02 μm(2)/s, n = 8) was not affected. Compared to control cells, a statistically significant increase of 17.5 ± 6.4% (n = 4, p < 0.05) and 24.2 ± 5.8% (n = 4, p < 0.001) in the cAMP formation was found for β-hederin pretreated HASM cells after stimulation with 10 μM of terbutaline and simultaneous stimulation with 10 μM terbutaline and 10 μM forskolin, respectively.
CONCLUSIONS:
Within this systematic study focusing on the influence of the ingredients of an ivy leaves dry extract on HASM cells it was possible to identify Beta-Hederin as further component presumably responsible for the β2-mimetic effects.
PLoS One. 2014 Mar 6;9(6):e90848.
The apoptotic effect of D Rhamnose β-hederin, a novel oleanane-type triterpenoid saponin on breast cancer cells.[Pubmed:
24603880 ]
There is growing interest in development of natural products as anti-cancer and chemopreventive agents. Many triterpenoids have been proved as potential agents for chemoprevention and therapy of breast cancer. Ginsenosides from ginseng, which mostly belong to dammarane-type triterpenoids, have gained great attention for their anti-breast cancer activity with diverse mechanisms. However, studies of other kinds of triterpenoid saponins on breast cancer are limited. Previously, we purified and identified a novel oleanane-type triterpene saponin named D Rhamnose Beta-Hederin (DRβ-H) from Clematis ganpiniana, a Chinese traditional anti-tumor herb.
METHODS AND RESULTS:
In the present study, DRβ-H showed strong inhibitory activity on the growth of various breast cancer cells and induced apoptosis in these cells. DRβ-H inhibited PI3K/AKT and activated ERK signaling pathway. PI3K inhibitor LY294002 synergistically enhanced DRβ-H-induced apoptosis whereas MEK inhibitor U0126 reduced the apoptosis rate. Moreover, DRβ-H regulated the ratio of pro-apoptotic and anti-apoptotic Bcl-2 family proteins. Furthermore, DRβ-H induced depolarization of mitochondrial membrane potential which released Apaf-1 and Cytochrome C from the inter membrane space into the cytosol, where they promoted caspase-9 and caspase-3 activation.
CONCLUSIONS:
This is the first report on the pro-apoptotic effects of DRβ-H, a novel oleanane-type triterpenoid saponin, on breast cancer cells and its comprehensive apoptosis pathways. It implied that oleanane-type triterpenoid saponin DRβ-H could be a promising candidate for chemotherapy of breast cancer.
Carbohydr Res. 2006 Jan 16;341(1):60-7.
Synthesis of beta-hederin and Hederacolchiside A1: triterpenoid saponins bearing a unique cytotoxicity-inducing disaccharide moiety.[Pubmed:
16297897]
A facile synthetic approach toward oleanolic acid glycoside bearing alpha-L-rhamnopyranosyl-(1-->2)-alpha-L-arabinopyranosyl moiety, a unique oligosaccharide that strongly induces antitumor activity of oleanane-type triterpenoid saponins, was developed.
METHODS AND RESULTS:
Based on this approach Beta-Hederin (oleanolic acid 3-O-alpha-L-rhamnopyranosyl-(1-->2)-alpha-L-arabinopyranoside) was efficiently prepared from oleanolic acid through stepwise glycosylation in linear eight steps with 52% overall yield, while Hederacolchiside A1 (oleanolic acid 3-O-alpha-L-rhamnopyranosyl-(1-->2)-[beta-D-glucopyranosyl-(1-->4)]-alpha-L-arabinopyranoside) in linear 13 steps with 20% overall yield.