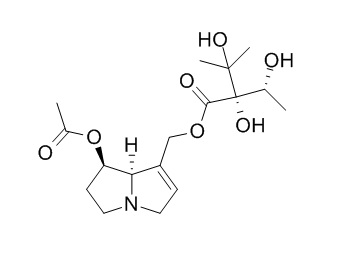
Uplandicine
Uplandicine exhibits antibacterial effects with a MIC of 1.7 mg/ml in E. coli.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Korean J Dent Mater.2018, 45(2):139-146
Foods.2021, 10(11):2627.
Int. J. of Food Properties2017, S108-S118
Chin J Pharm Anal.2019, 39(7):1217-1228
Primary and Industrial.2018, 52(11)
J Food Sci Technol.2022, 59(1):212-219.
Int J Biol Macromol.2020, 169:342-351
ACS Omega.2021, 6(36):23460-23474.
Phytomedicine.2024, 122:155065.
J Adv Res.2021, 35:245-257.
Related and Featured Products
Zeitschrift für Naturforschung C, 2016, 54(5-6): 295-300.
Pyrrolizidine alkaloids from Echium rauwolfii and Echium horridum (Boraginaceae).[Pubmed:
10431383]
METHODS AND RESULTS:
Echimidine was isolated from Echium rauwolfii and Echium horridum and identified by MS, 1H- and 13C NMR as a major alkaloid. In addition, structures of 12 minor alkaloids were inferred from GLC and GLC-MS analyses: 7-angeloylretronecine, 7-tigloylretronecine, lycopsamine, 7-acetyllycopsamine, Uplandicine, 7-angeloyllycopsamine, 7-tigloyllycopsamine, tigloyl isomer of echimidine, 7-angeloyl-9-(2-methylbutyryl)retronecine, 7-tigloyl-9-(2-methylbutyryl)retronecine, 7-angeloyl-9-(2,3-dihydroxybutyryl)retronecine, and 7-tigloyl-9-(2,3-dihydroxybutyryl)retronecine.
CONCLUSIONS:
Both species had similar alkaloid profiles. Alkaloid extracts exhibited antibacterial effects with a MIC of 1.7 mg/ml in E. coli. Microscopic examination of E. coli treated with different subtoxic alkaloid concentrations (13-52 micrograms/ml) revealed extensive filamentation.
J Agric Food Chem. 2005 Feb 9;53(3):594-600.
Pyrrolizidine alkaloids of Echium vulgare honey found in pure pollen.[Pubmed:
15686407]
METHODS AND RESULTS:
The pyrrolizidine alkaloids previously identified in floral honey attributed to Echium vulgare (Boraginaceae) have been detected (8000-14 000 ppm) in pure pollen collected from the anthers of Echium vulgare.
Pyrrolizidine alkaloids and/or their N-oxides were isolated from the aqueous acid extracts of pollen by use of strong cation-exchange, solid-phase extraction and identified by liquid chromatographic/mass spectrometric (LCMS) analysis.
CONCLUSIONS:
The pyrrolizidine alkaloids in the pollen are present mainly as the N-oxides. In addition to seven previously described pyrrolizidine alkaloids and/or their N-oxides (echimidine, acetylechimidine, Uplandicine, 9-O-angelylretronecine, echiuplatine, leptanthine, and echimiplatine), one unidentified (echivulgarine), but previously found in honey, and two previously undescribed (vulgarine and 7-O-acetylvulgarine) pyrrolizidine alkaloids and/or their N-oxides were identified in the pollen.
Tentative structures for these unidentified pyrrolizidine alkaloids are proposed on the basis of the mass spectrometric data and biogenetic considerations. The implications of these results for identifying the source and subsequent concentrations of pyrrolizidine alkaloids in honeys and commercial bee pollen are briefly discussed.