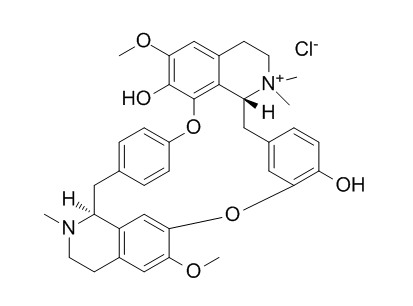
(+)-Tubocurarine chloride
Reference standards.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Food Chem.2023, 427:136647.
Adaptive Medicine 2020, 12(1): 4-10
Journal of Medicinal Food2023, Vol.26(10).
Evid Based Complement Alternat Med.2019, 2019:2135351
Vietnam Journal of Science2022, 64(2), 69-75.
Nutrients.2020, 12(5):1242.
Toxins (Basel).2023, 15(3):231.
Sci Rep.2023, 13(1):21690.
Food Chem.2024, 452:139555.
Biomed Pharmacother.2024, 179:117365.
Related and Featured Products
British Journal of Pharmacology, 1982, 75(3):503-512.
The ionization of morphine, hydroxyamphetamine and (+)-tubocurarine chloride and a new method for calculating zwitterion constants.[Reference:
WebLink]
METHODS AND RESULTS:
1 An improved method for estimating the zwitterion constants of phenolic amines is described which involves the exploratory least-squares fit of absorbance (at a suitable wavelength) to pH, starting with estimates of pK1 and pK2 obtained electrometrically. 2 With the method it is possible to see that hydroxyamphetamine (alpha-methyltyramine) has a higher zwitterion constant than tyramine and the zwitterion constants of both compounds are lower at 37 degree C than at 25 degree C. 3 The zwitterion constant of morphine is not reduced by raising the temperature from 25 degree to 37 degree C and the effect of temperature is much greater in compounds with a primary or secondary amino group than with those containing a tertiary amino group. Some zwitterions may be stabilized by hydration and their formation will be reduced by a rise in temperature which will break up water structure. 4 From electrometric titrations with (+)-Tubocurarine chloride in 0.1 M NaCl estimates of pK1, pK2 and pK3 were 7.6, 8.65 and 9.65 at 25 degree C and 7.4, 8.6 and 9.7 at 37 degree C, compared with 7.8, 8.85 and 9.75 given by Perrin (1980).
CONCLUSIONS:
However, the effects of pH on absorbance show that the phenolic groups lose a proton before the ammonium group so there is extensive zwitterion formation which is probably greater at 25 degree than at 37 degree C. the p-phenolic group (position 13) probably ionizes first with the phenate form stabilized by hydration involving water molecules and the protonated form of the (1-) ammonium group.