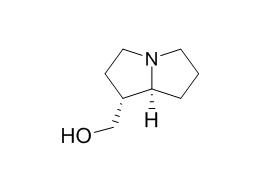
Trachelanthamidine
Reference standards.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Molecules.2017, 22(6)
Microchemical Journal2023, 194:109249
Molecules.2022, 27(21):7643.
Molecules.2023, 28(19):6775.
J Biomol Struct Dyn.2022, 1-21.
Korean J Pain.2021, 34(4):405-416.
ACS Pharmacol Transl Sci.2024, 7(2):395-405.
Evid Based Complement Alternat Med.2015, 2015:165457
Journal of Herbal Medicine2024, 48:100950
Malaysian Journal of Analytical Sciences2023, 27(4):840-848.
Related and Featured Products
Angew Chem Int Ed Engl. 2014 Nov 24;53(48):13196-200.
Organocatalytic asymmetric Mannich cyclization of hydroxylactams with acetals: total syntheses of (-)-epilupinine, (-)-tashiromine, and (-)-trachelanthamidine.[Pubmed:
25264221]
METHODS AND RESULTS:
An asymmetric, organocatalytic, one-pot Mannich cyclization between a hydroxylactam and acetal is described to provide fused, bicyclic alkaloids bearing a bridgehead N atom. Both aliphatic and aromatic substrates were used in this transformation to furnish chiral pyrrolizidinone, indolizidinone, and quinolizidinone derivatives in up to 89% yield and 97% ee.
CONCLUSIONS:
The total syntheses of (-)-epilupinine, (-)-tashiromine, and (-)-Trachelanthamidine also achieved to demonstrate the generality of the process.
J Org Chem. 2010 Jun 4;75(11):3578-86.
Asymmetric synthesis of 2-alkyl-substituted 2,5-dihydropyrroles from optically active aza-Baylis-Hillman adducts. Formal synthesis of (-)-trachelanthamidine.[Pubmed:
20465267]
A series of optically active 2-alkyl-substituted 2,5-dihydropyrroles were prepared via the asymmetric aza-Baylis-Hillman equivalent reaction and subsequent ring-closure metathesis reaction.
METHODS AND RESULTS:
Optically active aza-Baylis-Hillman adducts underwent a smooth two-step conversion to N-allyl-beta-amino-alpha-methylene esters in high yield, which gave chiral 2,5-dihydropyrroles, potential precursors for the aza-heterocyclic synthesis, almost quantitatively through RCM reaction catalyzed by Grubbs catalyst. The conversion was carried out without loss of the optical purity of the starting material. Synthetic application of the method to (-)-Trachelanthamidine was examined. Hydrogenation of 2,5-dihydropyrrole took place smoothly to give the corresponding 2,3-disubstituted pyrrolidine in good yield. The stereoselectivity of the hydrogenation was sensitive to the presence or absence of the protective group in the C2-side chain. The TBS-protected 2,5-dihydropyrrole gave a 1:1 mixture of the cis/trans isomers, while free alcohol afforded the trans-2,3-disubstituted pyrrolidine in a selectivity of 6:1. The formal synthesis of (-)-Trachelanthamidine was achieved in 11 steps from a chiral sulfinimine.
CONCLUSIONS:
This methodology provided a convenient procedure for the preparation of C2-alkyl-substituted 2,5-dihydropyroles with retention of high optical purity.