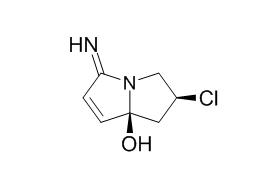
Clazamycin B
Clazamycin B is a novel pyrrolizidine antitumor antibiotic.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Chinese Journal of Hospital Pharmacy2020, 40(7)
Tea Res. Ins. Of China2017, 1-12
Molecules.2017, 22(3)
Front Plant Sci.2017, 8:723
Ecol Evol.2022, 12(11):e9459.
Evid Based Complement Alternat Med.2016, 2016:4357656
J Chromatogr B Analyt Technol Biomed Life Sci.2022, 1203:123307.
Braz J Med Biol Res.2021, 54(12):e11183.
Journal of Applied Pharmaceutical Science2022, 0(00), pp:001-007
J Liq Chromatogr R T2025, 2505536.
Related and Featured Products
Supplements to the 2nd Edition of Rodd's Chemistry of Carbon Compounds, 1975, Pages 15–69
Chapter 8 – Five-membered monoheterocyclic compounds: Alkaloids (continued): Pyrrolizidine alkaloids[Reference:
WebLink]
Additional plant families that are now known to contain pyrrolizidine alkaloids are the Apocynaceae, Celastraceae, Euphorbiaceae, Ranunculaceae, and Scrophulariaceae. The mass spectra of pyrrolizidine alkaloids, 13C nuclear magnetic resonance spectrometric assignments, ultraviolet spectra of 20 pyrrolizidine derivatives, and infrared spectra of 25 pyrrolizidine esters are the spectroscopic studies presented in this chapter.
METHODS AND RESULTS:
Analytical methods, such as ion exchange resins, high performance liquid chromatography (HPLC), gas–liquid chromatography–mass spectrometry, and reverse-phase HPLC are discussed. The chapter discusses the preparation and properties of necines of various kinds, such as monohydroxylated derivatives, unhydroxylated derivatives, dihydroxylated derivatives, and trihydroxylated derivatives. The chapter also illustrates the synthesis of necic acids that include C6-acids, C7-acids, C8-acids, C9-acids, and C10-acids. The structural confirmation of various compounds, such as nitropolyzonamine from the defensive secretions of the millipede Polyzonium rosalbum, peduncularine found in Aristotelia peduncularis, Clazamycin A and Clazamycin B isolated from Streptomyces species are characterized.
CONCLUSIONS:
The chapter discusses the toxic effects on humans caused by ingestion of pyrrolizidine alkaloids. Hepatotoxicity, carcinogenicity, and accidental poisoning occur on consumption of foodstuffs contaminated with seeds of various plants that produce pyrrolizidine alkaloids.
J Nat Prod. 1987 May-Jun;50(3):360-7.
Studies on the pyrrolizidine antitumor agent, clazamycin: interconversion of clazamycins A and B.[Pubmed:
3668555]
METHODS AND RESULTS:
Clazamycin, a novel pyrrolizidine antitumor antibiotic, exists in aqueous solution as a mixture of two epimers, clazamycin A and Clazamycin B [1A, 1B], the ratio of which is pH dependent. Several lines of evidence are presented, including the results of trapping experiments and a study demonstrating base promoted interconversion of the two forms, that implicate an azacyclooctenone species [3] as an intermediate in the interconversion process.
CONCLUSIONS:
This result supports a previous observation, that the C6a carbinolamidine hydroxyl of clazamycin is unreactive towards nucleophiles and may be significant in helping to elucidate the mechanism of action of this antibiotic.