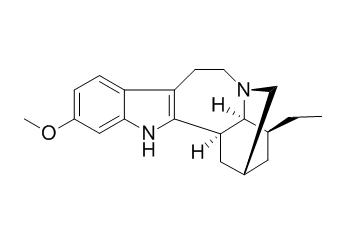
Tabernanthine
Tabernanthine shows affinity for opiate receptors, it can decrease morphine and cocaine self-administration in rats, it may be effective in treating addiction to opioid and stimulant drugs.Tabernanthine tartrate has peripheral cardiovascular effects in anaesthetized rats.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
J Neuroinflammation.2020, 17(1):75.
Front Nutr.2023, 10:1181135.
Phytomedicine.2023, 120:155063.
J Agric Food Chem.2020, 68(43):12164-12172.
Phytomedicine.2019, 59:152785
Pharmacogn Mag.2015, 11:S585-91
Life (Basel).2021, 11(7):616.
Cell Biochem Funct.2018, 36(6):303-311
Asian J Beauty Cosmetol2024, 22(1): 103-112.
Int J Cosmet Sci.2019, 41(1):12-20
Related and Featured Products
Brain Res. 1994 Sep 19;657(1-2):14-22.
Effects of iboga alkaloids on morphine and cocaine self-administration in rats: relationship to tremorigenic effects and to effects on dopamine release in nucleus accumbens and striatum.[Pubmed:
7820611]
Ibogaine, a naturally occurring alkaloid, has been claimed to be effective in treating addiction to opioid and stimulant drugs and has been reported to decrease morphine and cocaine self-administration in rats. The present study sought to determine if other iboga alkaloids, as well as the chemically related harmala alkaloid harmaline, would also reduce the intravenous self-administration of morphine and cocaine in rats.
METHODS AND RESULTS:
Because both ibogaine and harmaline induce tremors, an effect that may be causally related to neurotoxicity in the cerebellar vermis, the temorigenic activities of the other iboga alkaloids were assessed. Lastly, in view of the involvement of the dopaminergic mesolimbic system in the actions of drugs of abuse, the effects of some of the iboga alkaloids on extracellular levels of dopamine and its metabolites in the nucleus accumbens and striatum were determined. All of the tested alkaloids (i.e., ibogaine, Tabernanthine, R- and S-coronaridine, R- and S-ibogamine, desethylcoronaridine, and harmaline) dose-dependently (2.5-80 mg/kg) decreased morphine and cocaine intake in the hour after treatment; decreases in morphine and cocaine intake intake were also apparent the day after administration of some but not all of these alkaloids (i.e., ibogaine, Tabernanthine, desethylcoronaridine, and the R-isomers of coronaridine and ibogamine).
CONCLUSIONS:
In some rats, there were persistent decreases in morphine or cocaine intake for several days after a single injection or after two or three weekly injections of one or another of these alkaloids; R-ibogamine produced such effects more consistently than any of the other alkaloids.
Eur J Pharmacol. 1987 Aug 21;140(3):303-9.
Benzodiazepine receptors are involved in tabernanthine-induced tremor: in vitro and in vivo evidence.[Pubmed:
2820763]
Tabernanthine, an indol alkaloid, is structurally related to carbolines (harmane, harmaline) which, in vitro, displace specific flunitrazepam binding to brain benzodiazepine receptors.
METHODS AND RESULTS:
In vivo, both Tabernanthine and carbolines cause a fine general tremor, suggesting that a possible interaction with benzodiazepine receptors could be involved in the activity of Tabernanthine. This hypothesis was validated by the in vitro and in vivo antagonism of benzodiazepine by Tabernanthine. In vitro, Tabernanthine inhibited specific flunitrazepam binding in a competitive manner with an affinity (IC50 150 microM) in the same range as harmane. Tabernanthine appeared as a benzodiazepine receptor inverse agonist in a discriminant in vitro binding assay. In vivo, the time course of tremorigenic activity was related to the Tabernanthine concentration in brain (half-life = 2 h). Moreover, Tabernanthine-induced tremor was inhibited reversibly by flunitrazepam or by Ro-15 1788 (an antagonist of benzodiazepine-receptors).
CONCLUSIONS:
These results suggest that part of the action of Tabernanthine may be mediated by an interaction at the benzodiazepine receptor level.
Brain Res. 1992 Feb 7;571(2):242-7.
Mechanisms of action of ibogaine and harmaline congeners based on radioligand binding studies.[Pubmed:
1377086]
METHODS AND RESULTS:
Assays using radioligands were used to assess the actions of ibogaine and harmaline on various receptor types. Ibogaine congeners showed affinity for opiate receptors whereas harmaline and harmine did not. The Ki for coronaridine was 2.0 microM at mu-opiate receptors. The Kis for coronaridine and Tabernanthine at the delta-opiate receptors were 8.1 and 3.1 microM, respectively. Ibogaine, ibogamine, coronaridine and Tabernanthine had Ki values of 2.08, 2.6, 4.3 and 0.15 microM, respectively, for kappa-opiate receptors. Long-lasting, dose-dependent behavioral effects of ibogaine have been reported. The possibility that these effects were due to irreversible binding properties of ibogaine at kappa-receptors was considered; however, radioligand wash experiments showed a rapid recovery of radioligand binding after one wash. A voltage-dependent sodium channel radioligand demonstrated Ki values in the microM range for all drugs tested. Using radioligand binding assays and/or 36Cl- uptake studies, no interaction of ibogaine or harmaline with the GABA receptor-ionophore was found.
CONCLUSIONS:
The kappa-activity of ibogaine (or an active metabolite) may be responsible for its putative anti-addictive properties whereas the tremorigenic properties of ibogaine and harmaline may be due to their effects on sodium channels.
Arch Int Pharmacodyn Ther. 1985 Jul;276(1):60-72.
Peripheral cardiovascular effects of tabernanthine tartrate in anaesthetized rats.[Pubmed:
4051640]
METHODS AND RESULTS:
The peripheral cardiovascular effects of Tabernanthine tartrate have been studied in anaesthetized rats. Our results confirm that the bradycardic effect of Tabernanthine is not inhibited by vagotomy, atropine or propranolol. On the contrary, bivagotomy, atropine treatment, as well as carotid artery occlusion, potentiate the bradycardic effect of Tabernanthine. The same is true for its hypotensive action and can be explained by the suppression of a compensatory mechanism involving the central nervous system, the parasympathetic system and/or a baroreflex mechanism. In addition, domperidone and sulpiride, two dopaminolytic drugs, are able to potentiate the decrease in heart rate produced by Tabernanthine. In pithed rat, Tabernanthine 1 mg/kg, potentiates the increases in systolic blood pressure produced either by norepinephrine or serotonine; conversely the systolic blood pressure responses to angiotensin II are significantly inhibited by Tabernanthine 1 mg/kg.
CONCLUSIONS:
Thus, Tabernanthine appears to possess a complex cardiovascular mechanism of action, depending probably on a simultaneous stimulation of beta 2-vascular adrenoceptors and alteration of cellular movements of calcium. Part of the direct bradycardic effect, as well as the inhibition of the pressor responses of angiotensin II could be explained by a calcium antagonist action of the alcaloid.
Phytochemical Analysis, 1999, 10(2):60-63.
Droplet counter-current chromatography of indole alkaloids from Tabernaemontana hilariana[Reference:
WebLink]
This paper reports the separation of the indole alkaloids from the benzene extract of the root barks of Tabernaemontana hilariana (Apocynaceae).
METHODS AND RESULTS:
The crude alkaloid fraction was fractionated by droplet counter-current chromatography using a low polarity mixture (hexane:ethyl acetate:ethanol:water). Nine indole alkaloids (3-hydroxycoronaridine, coronaridine, voacangine, 3-(2-oxopropyl) coronaridine, voacangine hydroxyindolenine, ibogamine, voacangine pseudoindoxyl, coronaridine pseudoindoxyl and Tabernanthine) were identified using thin layer chromatography, gas chromatography coupled with mass spectrometry and nuclear magnetic resonance spectroscopy.