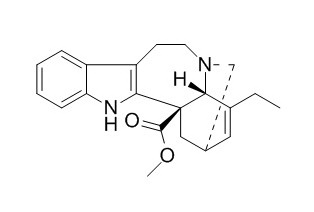
Catharanthine
Catharanthine inhibits nicotinic receptor mediated diaphragm contractions with IC50 of 59.6 μM, it dilates small mesenteric arteries and decreases heart rate and cardiac contractility by inhibition of voltage-operated calcium channels on vascular smooth muscle cells and cardiomyocytes.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Kaohsiung J Med Sci.2024, 40(3):280-290.
Food Chem Toxicol.2023, 176:113802.
Food Chem.2016, 191:81-90
Molecules.2024, 29(5):1050.
Molecules.2020, 25(11):2599.
Molecules.2019, 24(9):E1719
Acta Pharm Sin B.2015, 5(4):323-9.
J Integr Plant Biol.2023, 13564.
Preprints2021, doi:10.20944
Int J Biol Macromol.2020, 169:342-351
Related and Featured Products
J Pharmacol Exp Ther. 2013 Jun;345(3):383-92.
Catharanthine dilates small mesenteric arteries and decreases heart rate and cardiac contractility by inhibition of voltage-operated calcium channels on vascular smooth muscle cells and cardiomyocytes.[Pubmed:
23532933]
Catharanthine is a constituent of anticancer vinca alkaloids. Its cardiovascular effects have not been investigated.
METHODS AND RESULTS:
This study compares the in vivo hemodynamic as well as in vitro effects of Catharanthine on isolated blood vessels, vascular smooth muscle cells (VSMCs), and cardiomyocytes. Intravenous administration of Catharanthine (0.5-20 mg/kg) to anesthetized rats induced rapid, dose-dependent decreases in blood pressure (BP), heart rate (HR), left ventricular blood pressure, cardiac contractility (dP/dt(max)), and the slope of the end-systolic pressure-volume relationship (ESPVR) curve. Catharanthine evoked concentration-dependent decreases (I(max) >98%) in endothelium-independent tonic responses of aortic rings to phenylephrine (PE) and KCl (IC(50) = 28 μM for PE and IC(50) = 34 μM for KCl) and of third-order branches of the small mesenteric artery (MA) (IC(50) = 3 μM for PE and IC(50) = 6 μM for KCl). Catharanthine also increased the inner vessel wall diameter (IC(50) = 10 μM) and reduced intracellular free Ca(2+) levels (IC(50) = 16 μM) in PE-constricted MAs. Patch-clamp studies demonstrated that Catharanthine inhibited voltage-operated L-type Ca(2+) channel (VOCC) currents in cardiomyocytes and VSMCs (IC(50) = 220 μM and IC(50) = 8 μM, respectively) of MA. Catharanthine lowers BP, HR, left ventricular systolic blood pressure, and dP/dt(max) and ESPVR likely via inhibition of VOCCs in both VSMCs and cardiomyocytes.
CONCLUSIONS:
Since smaller vessels such as the third-order branches of MAs are more sensitive to VOCC blockade than conduit vessels (aorta), the primary site of action of Catharanthine for lowering mean arterial pressure appears to be the resistance vasculature, whereas blockade of cardiac VOCCs may contribute to the reduction in HR and cardiac contractility seen with this agent.
Biotechnol Prog. 2013 Jul-Aug;29(4):994-1001.
Interaction between abscisic acid and nitric oxide in PB90-induced catharanthine biosynthesis of catharanthus roseus cell suspension cultures.[Pubmed:
23554409]
Elicitations are considered to be an important strategy to improve production of secondary metabolites of plant cell cultures. However, mechanisms responsible for the elicitor-induced production of secondary metabolites of plant cells have not yet been fully elucidated.
METHODS AND RESULTS:
Here, we report that treatment of Catharanthus roseus cell suspension cultures with PB90, a protein elicitor from Phytophthora boehmeriae, induced rapid increases of abscisic acid (ABA) and nitric oxide (NO), subsequently followed by the enhancement of Catharanthine production and up-regulation of Str and Tdc, two important genes in Catharanthine biosynthesis. PB90-induced Catharanthine production and the gene expression were suppressed by the ABA inhibitor and NO scavenger respectively, showing that ABA and NO are essential for the elicitor-induced Catharanthine biosynthesis. The relationship between ABA and NO in mediating Catharanthine biosynthesis was further investigated. Treatment of the cells with ABA triggered NO accumulation and induced Catharanthine production and up-regulation of Str and Tdc. ABA-induced Catharanthine production and gene expressions were suppressed by the NO scavenger. Conversely, exogenous application of NO did not stimulate ABA generation and treatment with ABA inhibitor did not suppress NO-induced Catharanthine production and gene expressions.
CONCLUSIONS:
Together, the results showed that both NO and ABA were involved in PB90-induced Catharanthine biosynthesis of C. roseus cells. Furthermore, our data demonstrated that ABA acted upstream of NO in the signaling cascade leading to PB90-induced Catharanthine biosynthesis of C. roseus cells.
Biomed Chromatogr. 2015 Jan;29(1):97-102.
Liquid chromatography mass spectrometry simultaneous determination of vindoline and catharanthine in rat plasma and its application to a pharmacokinetic study.[Pubmed:
24828449]
Vinblastine and vincristine, both of which are bisindole alkaloids derived from vindoline and Catharanthine, have been used for cancer chemotherapy; their monomeric precursor molecules are vindoline and Catharanthine. A simple and selective liquid chromatography mass spectrometry method for simultaneous determination of vindoline and Catharanthine in rat plasma was developed.
METHODS AND RESULTS:
Chromatographic separation was achieved on a C18 (2.1 × 50 mm, 3.5 μm) column with acetonitrile-0.1% formic acid in water as mobile phase with gradient elution. The flow rate was set at 0.4 mL/min. An electrospray ionization source was applied and operated in positive ion mode; selective ion monitoring mode was used for quantification. Mean recoveries were in the range of 87.3-92.6% for vindoline in rat plasma and 88.5-96.5% for Catharanthine. Matrix effects for vindoline and Catharanthine were measured to be between 95.3 and 104.7%. Coefficients of variation of intra-day and inter-day precision were both <15%. The accuracy of the method ranged from 93.8 to 108.1%.
CONCLUSIONS:
The method was successfully applied in a pharmacokinetic study of vindoline and Catharanthine in rats. The bioavailability of vindoline and Catharanthine were 5.4 and 4.7%, respectively.