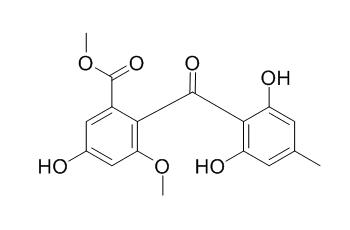
Sulochrin
Sulochrin shows antifungal and antibacterial activities, it can inhibit hepatitis C virus (HCV) infection in a dose-dependent manner without any apparent cytotoxicity up to 50 uM. Sulochrin has an inhibitory activity to eosinophil degranulation.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
J Mol Histol.2019, 50(4):343-354
Plants (Basel).2021, 10(6):1119.
Front Pharmacol.2025, 16:1611342.
Chinese Pharmacological Bulletin2019, 35(8):1120-1125
Scientific World Journal.2014, 2014:654193
Plants (Basel).2023, 12(5):1120.
Cell Rep.2022, 39(1):110643.
J Control Release.2024, 375:300-315.
Int J Mol Sci.2024, 25(1):616.
Journal of Research in Pharmacy.2022, 26(6):p1752-1757.
Related and Featured Products
Biochem Biophys Res Commun. 2013 Nov 1;440(4):515-20.
Specific inhibition of hepatitis C virus entry into host hepatocytes by fungi-derived sulochrin and its derivatives.[Pubmed:
24099774 ]
Hepatitis C virus (HCV) is a major causative agent of hepatocellular carcinoma. Although various classes of anti-HCV agents have been under clinical development, most of these agents target RNA replication in the HCV life cycle.
METHODS AND RESULTS:
To achieve a more effective multidrug treatment, the development of new, less expensive anti-HCV agents that target a different step in the HCV life cycle is needed. We prepared an in-house natural product library consisting of compounds derived from fungal strains isolated from seaweeds, mosses, and other plants. A cell-based functional screening of the library identified Sulochrin as a compound that decreased HCV infectivity in a multi-round HCV infection assay. Sulochrin inhibited HCV infection in a dose-dependent manner without any apparent cytotoxicity up to 50 μM. HCV pseudoparticle and trans-complemented particle assays suggested that this compound inhibited the entry step in the HCV life cycle.
CONCLUSIONS:
Sulochrin showed anti-HCV activities to multiple HCV genotypes 1a, 1b, and 2a. Co-treatment of Sulochrin with interferon or a protease inhibitor telaprevir synergistically augmented their anti-HCV effects.
Chem Pharm Bull (Tokyo). 1983 Dec;31(12):4543-8.
Studies on metabolites produced by Aspergillus terreus var. aureus. I. Chemical structures and antimicrobial activities of metabolites isolated from culture broth[Reference:
WebLink]
A fungus which was identified as Aspergillus terreus var. aureus showed antifungal and antibacterial activities.
METHODS AND RESULTS:
Through the investigation of its metabolites, six compounds (1-6) were isolated; emodin (1), dihydrogeodin (2), questin (3), Sulochrin (5), the new metabolite 2-(3-chloro-4-methyl-γ-resorcyloyl)-5-hydroxy-m-anisic acid methyl ester (4), which is a monochloro derivative of Sulochrin (5), and compound (6), the chemical structure of which has not yet been clarified.
CONCLUSIONS:
Dihydrogeodin (2), Sulochrin (5), 4 and 6 showed antifungal and antibacterial activities, and 6 has especially strong antifungal activity towards Trichophyton mentagrophytes.
Bioorg Med Chem Lett. 1999 Jul 19;9(14):1945-8.
Effects of ortho-substituent groups of sulochrin on inhibitory activity to eosinophil degranulation.[Pubmed:
10450959]
Sulochrin, a metabolite of fungi, has been shown to have an inhibitory activity to eosinophil degranulation.
METHODS AND RESULTS:
A series of Sulochrin derivatives substituted at ortho-positions to the 10-carbonyl group was examined the activity.
The importance of alkylester at C-6 position and several chemical properties of substituted groups at ortho-positions to exhibit activity are described.