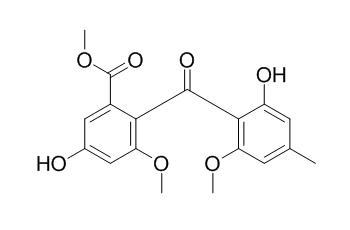
Monomethylsulochrin
Monomethylsulochrin shows antimicrobial activity against Escherichia coli, Pseudomonas aeruginosa, Bacillus subtilis and Staphylococcus aureus. It shows moderately inhibitory effects on the human bacterial pathogen Helicobacter pylori with the MIC value of 28.9+/-0.1 microM.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Int J Mol Sci.2019, 20(3):E651
J Cachexia Sarcopenia Muscle.2022, 13(6):3149-3162.
Eur J Pharmacol.2023, 951:175770.
Evid Based Complement Alternat Med.2020, 2020:1970349.
Korean J Dent Mater2020, 47(2):63-70.
J Agric Food Chem.2024, 72(15):8784-8797.
Appl. Sci. 2021, 11(8),3437.
Biomed Pharmacother.2022, 146:112497.
Appl. Sci. 2021, 11(17),7829
J Pharm Biomed Anal.2019, 164:119-127
Related and Featured Products
Chem Biodivers. 2010 Jan;7(1):216-20.
Benzophenones from Guignardia sp. IFB-E028, an endophyte on Hopea hainanensis.[Pubmed:
20087992 ]
METHODS AND RESULTS:
The first natural S-containing benzophenone dimer, named guignasulfide (3), was isolated from the culture of Guignardia sp. IFB-E028, an endophytic fungus residing in healthy leaves of Hopea hainanensis. Its structure was determined through correlative analyses of its MS, 1D- and 2D-NMR spectroscopic data. Two other known benzophenone derivatives, Monomethylsulochrin and rhizoctonic acid (1 and 2, resp.) were also isolated.
CONCLUSIONS:
Guignasulfide (3) was more active against the human liver cancer cell line HepG2 (IC(50) value: 5.2+/-0.4 microM) than metabolites 1 and 2 (IC(50) values: 63.5+/-0.6 and 60.2+/-0.5 microM); compounds 1-3 showed also moderately inhibitory effects on the human bacterial pathogen Helicobacter pylori with MIC values of 28.9+/-0.1, 60.2+/-0.4, and 42.9+/-0.5 microM, respectively.
J Antibiot (Tokyo). 2006 Jun;59(6):362-5.
A new diphenyl ether from marine-derived fungus Aspergillus sp. B-F-2.[Pubmed:
16915822 ]
METHODS AND RESULTS:
A new diphenyl ether dimethyl 2,3'-dimethylosoate (1) together with three known compounds Monomethylsulochrin (2), emodin (3), and questin (4) were isolated through bioassay-guided fractionations from the culture of a marine-derived fungus Aspergillus sp. B-F-2.
CONCLUSIONS:
The structures of these compounds were determined by spectroscopic methods. Cytotoxicities of compounds 1 and 2 against K562 cell line were preliminarily evaluated by the MTT method and flow cytometry.
An Acad Bras Cienc. 2013;85(4):1247-53.
Chemical constituents of Aspergillus sp EJC08 isolated as endophyte from Bauhinia guianensis and their antimicrobial activity.[Pubmed:
24141408 ]
The present work reports the isolation of five compounds from Aspergillus sp EJC08 isolated as endophytic from Bauhinia guianensis, a tipical plant of the Amazon.
METHODS AND RESULTS:
The compounds ergosterol (1), ergosterol peroxide (2), mevalolactone (3), Monomethylsulochrin (4) and trypacidin A (5) were isolated by chromatographic procedures and identified by spectral methods of 1D and 2D NMR and MS.
CONCLUSIONS:
Compounds 3, 4 and 5 were tested against Escherichia coli, Pseudomonas aeruginosa, Bacillus subtilis and Staphylococcus aureus and showed good activity.