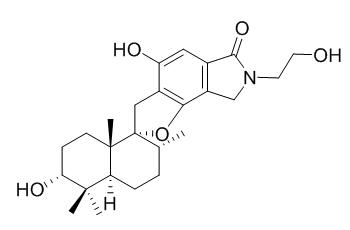
Stachybotramide
Stachybotramide preferentially stimulate the plasma cholesteryl ester transfer protein (CETP)-mediated transfer of cholesteryl esters (CE) from high density lipoprotein (HDL) to both very low density lipoprotein (VLDL) and low density lipoprotein (LDL).
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Phytochem Anal.2013, 24(5):493-503
Nutr Res Pract.2020, 14(3):203-217.
AMB Express2020. 10(1):126.
Appl Microbiol Biotechnol.2018, 102(12):5105-5120
Molecules.2022, 27(7):2116.
Biochem Pharmacol.2020, 178:114083
Res Rep Urol.2022, 14:313-326.
Evid Based Complement Alternat Med.2021, 2021:6687513.
Front Microbiol.2023, 14:1232039.
Food Chem.2022, 378:131975.
Related and Featured Products
Biochim Biophys Acta. 1995 Aug 24;1258(1):70-4.
Modulation of the plasma cholesteryl ester transfer by stachybotramide.[Pubmed:
7654783]
Plasma cholesteryl ester transfer protein (CETP), which mediates the transfer and exchange of neutral lipids (cholesteryl esters (CE) and triglycerides (TG) and phospholipids between plasma lipoproteins, plays an essential role in reverse cholesterol transport system.
METHODS AND RESULTS:
We have found that a fungal metabolite, Stachybotramide, modulates the activity of CETP. Stachybotramide stimulated the [14C]CE transfer from high density lipoprotein (HDL) to very low density lipoprotein (VLDL) and low density lipoprotein (LDL) by 1.3- to 1.5-fold at 0.2-0.5 mM. This stimulation was abolished in the presence of anti-CETP antibody. On the other hand, the transfer of [14C]CE from LDL and VLDL to HDL was slightly reduced by Stachybotramide at 0.5 mM. Unlike the transfer of [14C]CE, the transfer of [3H]TG from HDL was not significantly affected by Stachybotramide.
CONCLUSIONS:
These results suggest that Stachybotramide preferentially stimulate the CETP-mediated transfer of CE from HDL to both VLDL and LDL.
Mar Drugs. 2014 Apr 1;12(4):1924-38.
Spirocyclic drimanes from the marine fungus Stachybotrys sp. strain MF347.[Pubmed:
24694571 ]
METHODS AND RESULTS:
A novel spirocyclic drimane coupled by two drimane fragment building blocks 2 and a new drimane 1 were identified in mycelia and culture broth of Stachybotrys sp. MF347.
Their structures were established by spectroscopic means. This is the first example of spirocyclic drimane coupled by a spirodihydrobenzofuranlactam unit and a spirodihydroisobenzofuran unit; and the connecting position being N-C instead of an N and N connecting unit. Strain MF347 produced also the known spirocyclic drimanes stachybocin A (12) and stachybocin B (11) featured by two sesquiterpene-spirobenzofuran structural units connected by a lysine residue; the known spirocyclic drimanes chartarlactam O (5); chartarlactam K (6); F1839A (7); stachybotrylactam (8); Stachybotramide (9); and 2α-acetoxystachybotrylactam acetate (10); as well as ilicicolin B (13), a known sesquiterpene. The relative configuration of two known spirobenzofuranlactams (3 and 4) was determined. All compounds were subjected to biological activity tests.
CONCLUSIONS:
The spirocyclic drimane 2, 11, and 12, as well as the sesquiterpene 13, exhibited antibacterial activity against the clinically relevant methicillin-resistant Staphylococcus aureus (MRSA).