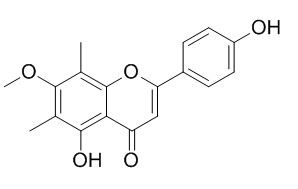
Sideroxylin
Sideroxylin can synergistically enhance the antimicrobial activity of the alkaloid berberine (also a constituent of H. canadensis) against Staphylococcus aureus by inhibition of the NorA multidrug resistance pump.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Separations2023, 10(7), 411.
Herbal Formula Science2024, 32(3):203-221
J Chromatogr Sci.2020, 58(6):485-493.
bioRxiv2021, 462065.
Front Pharmacol.2021, 12:615157.
Clin Exp Pharmacol Physiol.2020, doi: 10.1111
J Agric Food Chem.2024, 72(45):25219-25228.
Int Immunopharmacol. 2020, 83:106403.
Anticancer Res.2021, 41(3):1357-1364.
J Pharm Biomed Anal.2018, 151:32-41
Related and Featured Products
J Nat Prod. 2011 Jul 22;74(7):1621-9.
Synergy-directed fractionation of botanical medicines: a case study with goldenseal (Hydrastis canadensis).[Pubmed:
21661731]
It is often argued that the efficacy of herbal medicines is a result of the combined action of multiple constituents that work synergistically or additively. Determining the bioactive constituents in these mixtures poses a significant challenge.
METHODS AND RESULTS:
We have developed an approach to address this challenge, synergy-directed fractionation, which combines comprehensive mass spectrometry profiling with synergy assays and natural products isolation. The applicability of synergy-directed fractionation was demonstrated using the botanical medicine goldenseal (Hydrastis canadensis) as a case study. Three synergists from goldenseal were identified, Sideroxylin, 8-desmethyl-Sideroxylin, and 6-desmethyl-Sideroxylin. These flavonoids synergistically enhance the antimicrobial activity of the alkaloid berberine (also a constituent of H. canadensis) against Staphylococcus aureus by inhibition of the NorA multidrug resistance pump. The flavonoids possess no inherent antimicrobial activity against S. aureus; therefore, they could have been missed using traditional bioactivity-directed fractionation. The flavonoid synergists are present at higher concentration in extracts from H. canadensis leaves, while the antimicrobial alkaloid berberine is present at higher levels in H. canadensis roots.
CONCLUSIONS:
Thus, it may be possible to produce an extract with optimal activity against S. aureus using a combination of goldenseal roots and leaves.
J Nat Prod. 2015 Jul 24;78(7):1748-51.
An Isoflavone from Leiophyllum buxifolium and Its Antiproliferative Effect.[Pubmed:
26086179]
METHODS AND RESULTS:
A new C-methylisoflavone, isoSideroxylin (1), and a known C-methylflavone, Sideroxylin (2), were isolated from the EtOAc extract of the leaves of Leiophyllum buxifolium. The two compounds were evaluated with the sulforhodamine B assay for their antiproliferative effects against ER(-) MDA-MB-231 and ER(+) MCF-7 breast cancer cell lines and the NIH3T3 mouse fibroblast cell line.
CONCLUSIONS:
IsoSideroxylin (1) displayed a selective antiproliferative effect against MDA-MB-231 cells.
Planta Med. 2010 Jun;76(9):863-8.
C-methylflavonoids isolated from Callistemon lanceolatus protect PC12 cells against Abeta-induced toxicity.[Pubmed:
20101562]
Increased beta-amyloid (Abeta) production and its aggregation to the oligomeric state is considered to be a major cause of Alzheimer's disease (AD). Therefore, reducing Abeta-induced neurotoxicity could provide a suitable means of prevention or intervention in the disease course of AD. The neuroprotective effects of isolates from Callistemon lanceolatus DC. (Myrtaceae) against Abeta were evaluated using PC12 cells.
METHODS AND RESULTS:
To evaluate the effects of Abeta on apoptotic cell death and the effects of Bcl-2 family proteins and caspase-3, TUNEL assays and Western blotting were performed, respectively. Substantial fractionation and purification of the EtOAc-soluble extract of the aerial parts of C. lanceolatus afforded six flavonoids, 4',5-dihydroxy-6,8-dimethyl-7-methoxyflavanone (1), eucalyptin (2), 8-demethyleucalyptin (3), Sideroxylin (4), syzalterin (5), and quercetin (6). Compounds 1, 5, and 6 were found to protect PC12 cells effectively against Abeta-induced toxicity. In particular, compound 1 showed the most promising neuroprotective effect with an ED (50) value of 6.7 microM in terms of decreasing Abeta-induced apoptotic cell death, and this was accompanied by a decrease in caspase-3 activation and an increase in Bcl-2/Bax ratio.
CONCLUSIONS:
These results suggest that compound 1 could be developed as a candidate anti-AD agent due to its attenuation of Abeta-induced apoptotic cell death.