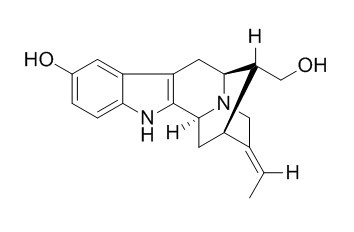
Sarpagine
Sarpagine is effective in reversing multidrug-resistance (MDR) in vincristine-resistant KB cells.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
J Ethnopharmacol.2019, 236:31-41
Journal of Medical Sciences2024, 44(5):p 222-227.
Int J Biol Macromol.2019, 126:653-661
Chem Biodivers.2023, 20(10):e202300741.
Evid Based Complement Alternat Med.2021, 8855980.
Metabolites.2019, 9(11):E271
Viruses.2017, 9(10)
Antioxidants (Basel).2022, 11(12):2496.
J Agric Food Chem.2023, 71(47):18510-18523.
Korean J. Medicinal Crop Sci2021, 10:345-352.
Related and Featured Products
Phytochemistry. 2014 Feb;98:204-15.
Macroline, akuammiline, sarpagine, and ajmaline alkaloids from Alstonia macrophylla.[Pubmed:
24342109]
METHODS AND RESULTS:
A total of seventeen alkaloids, comprising six macroline (including alstofolinine A, a macroline indole incorporating a butyrolactone ring-E), two ajmaline, one Sarpagine, and eight akuammiline alkaloids, were isolated from the stem-bark and leaf extracts of the Malayan Alstonia macrophylla. The structure and relative configurations of these alkaloids were established using NMR, MS and in several instances, confirmed by X-ray diffraction analysis.
CONCLUSIONS:
Six of these alkaloids were effective in reversing multidrug-resistance (MDR) in vincristine-resistant KB cells.
Angew Chem Int Ed Engl. 2015 Jan 2;54(1):315-7.
Enantioselective, protecting-group-free total synthesis of sarpagine alkaloids--a generalized approach.[Pubmed:
25346454]
METHODS AND RESULTS:
A generalized synthetic access to Sarpagine alkaloids through a joint synthetic sequence has been accomplished. Its applicability is showcased by the enantioselective total syntheses of vellosimine (1), N-methylvellosimine (3), and 10-methoxyvellosimine (8). The synthetic sequence is concise (eight steps) from known compound 13, and requires no protecting groups. The indole heterocycle was introduced in the last step. This strategy allows access to Sarpagine alkaloids through a shared synthetic route leading to precursor 10, which we term "privileged intermediate".
Starting from this intermediate, all Sarpagine alkaloids can be synthesized using phenylhydrazines with different substitution patterns (15-17).
CONCLUSIONS:
Our approach brings about the advantage, that synthesis optimization only needs to be performed once for many natural products. The key features of the synthesis are a [5+2]-cycloaddition and a ring enlargement.