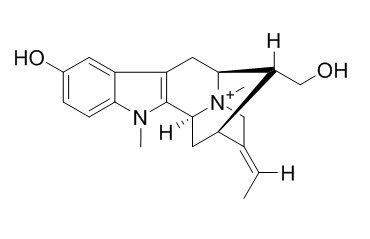
N-Methylsarpagine methosalt
Reference standards.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Korean Journal of Pharmacognosy.2019, 50(1):65-71
J Agric Food Chem.2024, 72(40):22237-22249.
Journal of Plant Growth Regulation2022, 10705-2.
Agriculture2024, 14(12), 2301
Plant Commun.2024, 5(10):101005.
Korean J. Food Preserv.2023, 30(4):663-668.
Mol Neurobiol.2021, 58(8):3665-3676.
Planta Med.2024, 2328-2750
Int J Mol Sci.2023, 24(18):13713.
Nat Commun.2025, 16(1):4121.
Related and Featured Products
Journal of organic chemistry, 2003, 34(49):6279.
The enantiospecific, stereospecific total synthesis of the ring-A oxygenated sarpagine indole alkaloids (+)-majvinine, (+)-10-methoxyaffinisine, and (+)-N(a)-methylsarpagine, as well as the total synthesis of the alstonia bisindole alkaloid macralstonidine.[Pubmed:
12895062 ]
METHODS AND RESULTS:
The first stereospecific, enantiospecific total synthesis of the ring-A oxygenated sarpagine indole alkaloids (+)-N(a)-methylsarpagine (N-Methylsarpagine methosalt, 8), (+)-majvinine (14), and (+)-10-methoxyaffinisine (49), as well as the first total synthesis of the Alstonia bisindole alkaloid macralstonidine (9), has been accomplished. This approach employed the Schöllkopf chiral auxiliary for the stereospecific construction of the desired d-(+)-tryptophan unit required for the asymmetric Pictet-Spengler reaction. In addition, the strategy was doubly convergent for the enolate-mediated Pd(0) coupling process and the asymmetric Pictet-Spengler reaction can be employed to synthesize both macroline (2) and N(a)-methylsarpagine (8), the coupling of which provides macralstonidine (9).
CONCLUSIONS:
This approach to ring-A substituted alkoxyindole alkaloids should find wide application for the synthesis of other alkaloids for it is stereospecific and either enantiomer can be prepared with ease.