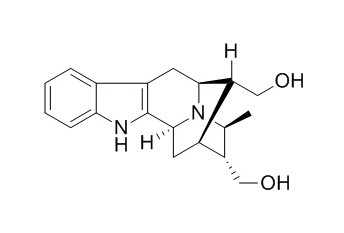
Dihydroperaksine
Reference standards.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Int J Mol Sci.2019, 21(1):E265
Bull.Natl.Mus.Nat.Sci.,Ser.B.2024, 50(2):79ĘC86
Nutr Res Pract.2023, 17(4):670-681.
Food Sci Nutr.2023, 00:1-10.
Biomol Ther (Seoul).2024, 32(5):546-555.
Chem Biol Interact.2018, 283:59-74
J of Archaeological Science:Reports2024, 53:104298
Foods.2023, 12(19):3647.
Journal of Life Science2018, 917-922
Food Chem.2024, 452:139555.
Related and Featured Products
J Org Chem. 2014 Nov 7;79(21):10030-48.
General strategy for synthesis of C-19 methyl-substituted sarpagine/macroline/ajmaline indole alkaloids including total synthesis of 19(S),20(R)-dihydroperaksine, 19(S),20(R)-dihydroperaksine-17-al, and peraksine.[Pubmed:
25247616]
METHODS AND RESULTS:
A detailed account of the development of a general strategy for synthesis of the C-19 methyl-substituted alkaloids including total synthesis of 19(S),20(R)-Dihydroperaksine-17-al (1), 19(S),20(R)-Dihydroperaksine (2), and peraksine (6) is presented.
Efforts directed toward the total synthesis of macrosalhine chloride (5) are also reported. Important to success is the sequence of chemical reactions which include a critical haloboration reaction, regioselective hydroboration, and controlled oxidation (to provide sensitive enolizable aldehydes at C-20). In addition, the all-important Pd-catalyzed ╬▒-vinylation reaction has been extended to a chiral C-19 alkyl-substituted substrate for the first time.
CONCLUSIONS:
Synthesis of the advanced intermediate 64 completes an improved formal total synthesis of talcarpine (26) and provides a starting point for synthesis of macroline-related alkaloids 27-31.
Similarly, extension of this synthetic strategy in the ring A oxygenated series should provide easy access to the northern hemisphere 32b of the bisindoles angustricraline, alstocraline, and foliacraline (Figure 4 ).
Org Lett. 2011 Oct 7;13(19):5216-9.
Regiospecific, enantiospecific total synthesis of C-19 methyl substituted sarpagine alkaloids dihydroperaksine-17-al and dihydroperaksine.[Pubmed:
21877687]
METHODS AND RESULTS:
The optically active tetracyclic ketone 8 was converted into the pentacylic core 14 of the C-19 methyl substituted N(a)-H sarpagine and ajmaline alkaloids via a critical haloboration reaction. The ketone 14 was then employed in the total synthesis of 19(S),20(R)-Dihydroperaksine-17-al (1) and 19(S),20(R)-Dihydroperaksine (2).
CONCLUSIONS:
The key regioselective hydroboration and controlled oxidation-epimerization sequence developed in this approach should provide a general method to functionalize the C(20)-C(21) double bond in the ajmaline-related indole alkaloids.