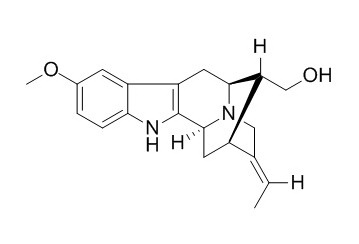
Lochnerine
Lochnerine shows potent vasorelaxant activity, it also shows some antitumor activity, it can bring about complete inhibition of cell growth in P388 leukemia cells in vitro.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
J AOAC Int.2023, 106(1):56-64.
Int Immunopharmacol.2023, 125:111175.
Biol Pharm Bull.2017, 40(6):797-806
Konkuk University2023, 29:4634721
JABS2020, 14:2(2020)
Korean J. Food Preserv. 2021, 28(6):846-856.
Plant Foods Hum Nutr.2020, 10.1007
Int J Nanomedicine.2022, 17:6513-6525.
International. J. of Food Properties 2017, 20:S131-S140
J. of Agricultural Science2015, 1916-9760
Related and Featured Products
Jpn J Cancer Res. 1986 Feb;77(2):197-204.
Non-antitumor vinca alkaloids reverse multidrug resistance in P388 leukemia cells in vitro.[Pubmed:
3082832]
Twelve monomeric or dimeric alkaloids from Vinca rosea Linn., which had been reported to have little or no antitumor activity, were investigated to determine their combined effects with either vincristine or daunorubicin on in vitro cell growth of a P388 subline resistant to vincristine and cross-resistant to anthracyclines.
METHODS AND RESULTS:
We found that the combinations at subcytotoxic concentrations induced significant growth inhibition of the resistant cells, but not of the sensitive cells. Of the alkaloids examined, catharine, vindoline, catharanthine, vincarodine, and Lochnerine were able to bring about complete inhibition of cell growth. Further in vitro study using vindoline revealed that at 10 micrograms/ml it was able to completely reverse not only resistance to vincristine but also cross-resistance to vinblastine, daunorubicin, and adriamycin. In addition, we found that vinca alkaloids active in reversing resistance possess potent activities to enhance the net uptake of not only vincristine but also daunorubicin by the resistant cells, and this effect was proved to result from their inhibitory action on the active efflux process.
CONCLUSIONS:
These results provide further support for our hypothesis that both anthracyclines and vinca alkaloids can inhibit their own efflux process by interacting with the cell membrane, and this similarity provides a basis for their reciprocal cross-resistance, irrespective of their different chemical structures.
J Nat Med. 2013 Jan;67(1):9-16.
Vasorelaxant activity of indole alkaloids from Tabernaemontana dichotoma.[Pubmed:
22350216]
The aim of this study was to search for bioactive natural products from medicinal plants targeting vasorelaxant activity and we found the methanol extract from bark of Tabernaemontana dichotoma showed vasorelaxant activity on rat aorta.
METHODS AND RESULTS:
We isolated eight indole alkaloids including 10-methoxyalstonerine (1), a new macroline type indole alkaloid, from bark of T. dichotoma. These were respectively identified as 10-methoxyaffinisine (2), Lochnerine (3), cathafoline (4), (-)-alstonerine (5), 19,20-dehydro-10-methoxytalcarpine (6), alstonisine (7), and alstonal (8) based on spectroscopic analysis. Among them, sarpagine type (2 and 3), akuammiline type (4), and macroline oxindole type (7 and 8) showed potent vasorelaxant activity. Mechanism of action on vasorelaxant activity of 10-methoxyaffinisine (2), cathafoline (4), and alstonisine (7) was clarified.
CONCLUSIONS:
Effects of 10-methoxyaffinisine (2), cathafoline (4), and alstonisine (7) were partially mediated the NO release from endothelial cells. Furthermore, 10-methoxyaffinisine (2) and alstonisine (7) attribute to the inhibitory effect of VDC and ROC, and cathafoline (4) have inhibitory effect on Ca(2+) influx via ROC. In addition, 10-methoxyaffinisine (2) as a major compound from bark of T. dichotoma showed hypotensive effect on normotensive rats in vivo.
J Org Chem. 2013 Jul 5;78(13):6471-87.
Stereospecific approach to the synthesis of ring-A oxygenated sarpagine indole alkaloids. Total synthesis of the dimeric indole alkaloid P-(+)-dispegatrine and six other monomeric indole alkaloids.[Pubmed:
23721107]
METHODS AND RESULTS:
The first regio- and stereocontrolled total synthesis of the bisphenolic, bisquaternary alkaloid (+)-dispegatrine (1) has been accomplished in an overall yield of 8.3% (12 reaction vessels) from 5-methoxy-d-tryptophan ethyl ester (17). A crucial late-stage thallium(III) mediated intermolecular oxidative dehydrodimerization was employed in the formation of the C9-C9' biaryl axis in 1. The complete stereocontrol observed in this key biaryl coupling step is due to the asymmetric induction by the natural sarpagine configuration of the monomer Lochnerine (6) and was confirmed by both the Suzuki and the oxidative dehydrodimerization model studies on the tetrahydro β-carboline (35).
CONCLUSIONS:
The axial chirality of the Lochnerine dimer (40) and in turn dispegatrine (1) was established by X-ray crystallography and was determined to be P(S). Additionally, the first total synthesis of the monomeric indole alkaloids (+)-spegatrine (2), (+)-10-methoxyvellosimine (5), (+)-Lochnerine (6), lochvinerine (7), (+)-sarpagine (8), and (+)-lochneram (11) were also achieved via the common pentacyclic intermediate 16.
Angelol M
Catalog No: CFN95060
CAS No: 1092952-64-1
Price: $318/10mg
5-Hydroxy-1-(4-hydroxyphenyl)-7-phenyl-3-heptanone (AO 2210)
Catalog No: CFN95137
CAS No: 105955-04-2
Price: $318/10mg
(1E)-3-methoxy-8,12-epoxygermacra-1,7,10,11-tetraen-6-one
Catalog No: CFN95219
CAS No: 1393342-06-7
Price: $413/5mg
Methylgomisin O
Catalog No: CFN95236
CAS No: 1276654-07-9
Price: $318/5mg
2-Phenylethyl-beta-glucopyranoside
Catalog No: CFN95429
CAS No: 18997-54-1
Price: $318/10mg
Kaempferol 3,5-O-diglucoside
Catalog No: CFN95487
CAS No: 205103-97-5
Price: $318/10mg
Isorhamnetin 3,5-O-diglucoside
Catalog No: CFN95488
CAS No: 2035413-03-5
Price: $318/10mg
12beta-Acetoxy-3,7,11,15,23-pentaoxo-lanost-8,20-dien-26-oic acid
Catalog No: CFN95505
CAS No: 1309931-91-6
Price: $318/5mg
Sophoraflavone A
Catalog No: CFN95510
CAS No: 105594-08-9
Price: $318/10mg
Mahuannin B
Catalog No: CFN95554
CAS No: 82796-37-0
Price: $318/5mg