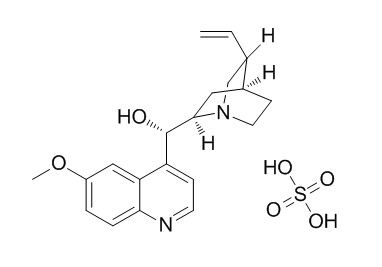
Quinidine sulfate
Quinidine sulfate has antiarrhythmic efficacy. Quinidine is a non-specific ionic channel blocker that inhibits all the membrane currents in the atrioventricular node including the acetylcholine-activated K+ current.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Molecules.2023, 28(19):6767.
Food Chem.2018, 252:207-214
Journal of Chromatography A2020, 460942
Chem Biol Interact.2016, 260:168-175
J Microbiol Biotechnol.2022, 32(2):141-148.
Nutrients.2023, 15(6):1417.
Pharmacognosy Magazine2017, 13(52):868-874
J Agric Food Chem.2024, acs.jafc.4c01387.
Exp Neurobiol.2018, 27(3):200-209
J Biomol Struct Dyn.2022, 5;1-17.
Related and Featured Products
Archiv Für Experimentelle Pathologie Und Pharmakologie, 1990, 341(6):517-524.
Anticholinergic action of quinidine sulfate in the rabbit atrioventricular node.[Reference:
WebLink]
METHODS AND RESULTS:
Anticholinergic action of Quinidine sulfate was electrophysiologically studied by recording spontaneous action potentials and membrane current of the rabbit atrioventricular node. In the presence of 0.1 mumol/l carbachol, the spontaneous activity of the atrioventricular nodal preparations was markedly inhibited, whereas subsequent addition of 1, 5 and 20 mumol/l quinidine restored automaticity in a concentration-dependent manner. In some preparations, quinidine at concentrations of 5 mumol/l and higher slowed the spontaneous activity by its direct membrane action even in the presence of carbachol. The dose-response curve for acetylcholine action on the spontaneous firing frequency showed that one molecule of acetylcholine bound to one muscarinic receptor of the atrioventricular node cell (Hill coefficient = 1.2). A parallel shift of this curve towards higher acetylcholine concentrations was observed at 0.03, 0.1 and 0.3 mumol/l but not at 1 and 3 mumol/l quinidine, suggesting a noncompetitive antagonism of quinidine against acetylcholine. Voltage clamp experiments revealed that 5 mumol/l quinidine reduced the slow inward current, hyperpolarization-activated inward current, and delayed rectifying K+ current, through its membrane actions. Quinidine at this concentration almost completely suppressed the acetylcholine-activated K+ current, which showed a relaxation phenomenon. Hence, the direct blockage of the acetylcholine-activated K+ current by quinidine was considered responsible for the anticholinergic action of this drug.
CONCLUSIONS:
We conclude that quinidine is a non-specific ionic channel blocker that inhibits all the membrane currents in the atrioventricular node including the acetylcholine-activated K+ current.
American journal of cardiology, 1985, 56(10):581-585.
Comparative efficacy and safety of oral tocainide and quinidine for benign and potentially lethal ventricular arrhythmias.[Reference:
WebLink]
METHODS AND RESULTS:
The antiarrhythmic efficacy and safety of oral tocainide hydrochloride and Quinidine sulfate were compared in a double-blind, 3-center, parallel trial involving 133 patients with benign and potentially lethal ventricular arrhythmias. Baseline demographic, etiologic, functional and ventricular arrhythmia data were not significantly different between the 2 groups. Two weeks of an initial placebo period were followed by 8 weeks of active drug treatment, concluding with 4 weeks of washout. Frequent 24-hour ambulatory electrocardiographic monitoring was used to judge efficacy. Ten of 27 patients (37%) receiving tocainide and 12 of 24 patients (50%) receiving quinidine had a 75% reduction with drug treatment compared with the initial placebo period (p >0.25). Total abolition of ventricular tachycardia occurred in 6 of 16 patients (37%) receiving tocainide and 6 of 13 patients (43%) receiving quinidine (p >0.25). Conditions that required discontinuation of therapy occurred in 18 of 67 patients (27%) receiving tocainide and 16 of 66 (24%) receiving quinidine (difference not significant). More patients had dizziness during tocainide treatment and diarrhea during quinidine treatment. Quinidine caused a prolongation in the QT interval (0.03 second); tocainide caused a slight reduction (0.01 second). No important changes in vital signs or laboratory measurements were observed in left ventricular ejection fraction when measured.
CONCLUSIONS:
Thus, tocainide, the new oral analog of lidocaine, appears to be as safe as quinidine but is slightly less effective in suppressing ventricular arrhythmias.