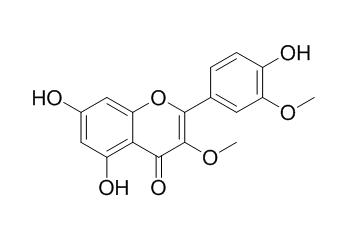
Quercetin 3,3'-dimethyl ether
Quercetin 3,3'-dimethyl ether may have moderate estrogenic activity.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Tropical Journal of Pharmaceutical Research 2021, 20(6):1165-1170.
J Cachexia Sarcopenia Muscle.2022, 13(6):3149-3162.
J Appl Pharm Sci.2022, 12(04):044-053
Nanjing University of Chinese Medicine2022, 345930.
Front. Plant Sci.2022, 13:757852.
Cell Death Discov.2023, 9(1):350.
J Sep Sci.2018, 41(7):1682-1690
Int J Mol Sci.2020, 21(24):9369.
Nutrients2023, 15(18), 4016.
J Clin Med.2019, 8(10):E1664
Related and Featured Products
Jaceidin triacetate
Catalog No: CFN99482
CAS No: 14397-69-4
Price: Inquiry(manager@chemfaces.com)
Chrysosplenetin
Catalog No: CFN97026
CAS No: 603-56-5
Price: $138/20mg
4',5,7-Trihydroxy 3,3',6,8-tetramethoxyflavone
Catalog No: CFN70415
CAS No: 58130-91-9
Price: Inquiry(manager@chemfaces.com)
3',4',7,8-Tetramethoxyflavone
Catalog No: CFN70432
CAS No: 65548-55-2
Price: $100/20mg
4'-hydroxy-6,7,8,3'-tetramethoxyflavonol
Catalog No: CFN91846
CAS No: 1879030-01-9
Price: $318/5mg
5,7,3',4'-Tetramethoxyflavone
Catalog No: CFN91116
CAS No: 855-97-0
Price: $30/20mg
Retusin
Catalog No: CFN89520
CAS No: 1245-15-4
Price: $158/10mg
Quercetin 3,5,7,3,4-pentamethyl ether
Catalog No: CFN70262
CAS No: 1247-97-8
Price: Inquiry(manager@chemfaces.com)
Artemetin
Catalog No: CFN98731
CAS No: 479-90-3
Price: $80/20mg
Artemetin acetate
Catalog No: CFN97530
CAS No: 95135-98-1
Price: Inquiry(manager@chemfaces.com)
Phytochemistry. 2002 Jun;60(4):385-7.
A benzoquinone and flavonoids from Cyperus alopecuroides.[Pubmed:
12031430]
METHODS AND RESULTS:
A benzoquinone, named alopecuquinone, was isolated from the ethanol extract of the inflorescences of Cyperus alopecuroides.
Its structure was primarily elucidated by spectroscopic analysis including 1H, 13C NMR, APT, HMQC, 1H-1H COSY and CIMS. The known flavonoids, vicenin 2, orientin, diosmetin, Quercetin 3,3'-dimethyl ether and its 3,4'-dimethyl ether, were also isolated and characterized.
CONCLUSIONS:
The ethanol extract of the plant material showed moderate estrogenic activity using a strain of Saccharomyces cerevisiae.
Phytochemistry. 2016 Dec;132:76-85.
Toxic aromatic compounds from fruits of Narthecium ossifragum L.[Pubmed:
27720435 ]
The intake of Narthecium ossifragum, commonly known as bog asphodel, has been associated with toxic effects observed in sheep for centuries. Although the plant has been studied for five centuries little is known about its chemical constituents.
METHODS AND RESULTS:
Six previously undescribed natural products, naringenin(3 → 6″)luteolin, naringenin(3 → 6″)chrysoeriol, liovil 4-O-β-glucopyranoside, 2,6-dimethoxy cinnamic acid, (E)-4-(3-hydroxy-2,2-dimethylchroman-6-yl)but-3-en-2-one and (E)-4-(4-(((E)-4-hydroxy-3-methylbut-2-en-1-yl)oxy)phenyl)but-3-en-2-one, have been identified from fruits of N. ossifragum for the first time. In addition, the rare natural product 4-hydroxy-3-(3-methylbut-2-enyl)benzaldehyde and the five known compounds 4-hydroxycinnamic acid, Quercetin 3,3'-dimethyl ether, quercetin 3,7-dimethyl ether, chrysoeriol 7-O-β-glucopyranoside and the di-C-glycosylflavone isoschaftoside were all characterized for the first time from the fruits of N. ossifragum.
CONCLUSIONS:
The discovery of sufficient amounts of 4-hydroxy-3-(3-methylbut-2-enyl)benzaldehyde in fresh plant material of N. ossifragum to allow complete structure elucidation by NMR and HRMS supports the possibility that fungi associated with N. ossifragum may be able to produce enough toxins to play a significant role in the pathogenicity of N. ossifragum.