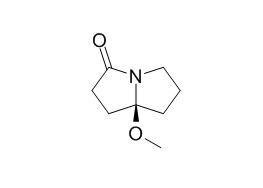
Pyrrolam B
Pyrrolam B is a natural product from Streptomyces olivaceus.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Biochem Biophys Res Commun.2017, 494(3-4):587-593
Biomedicine & Pharmacotherapy2020, 125:109950
World J Mens Health.2019, 10.5534
Phytomedicine.2022, 100:154058.
Journal of Ginseng Research2021, 3 June.
Molecules.2024, 29(22):5261.
Front Cell Dev Biol.2021, 9:588093.
Korean Journal of Pharmacognosy2018, 49(4):349-361
J Food Sci Technol.2022, 59(1):212-219.
ACS Nano.2023, 17(11):9972-9986.
Related and Featured Products
Author version: Synthesis, 2012,44; 2373-2681.
Pyrrolizidine Alkaloids Pyrrolams A-D: Survey on Synthetic Efforts, Biological Activities, and Studies on their Stability[Reference:
WebLink]
Pyrrolam A is a microbial metabolite, structurally related to plant alkaloids of the necine-type, and was isolated from the bacterial strain of Streptomyces olivaceus along with the related alkaloids Pyrrolam B, pyrrolam C, and pyrrolam D .
METHODS AND RESULTS:
The synthesis of (S)- and (R)-pyrrolam A has attracted the attention of chemists in recent years, with 10 syntheses reported to date. The reported routes utilize the advantages of chiral proline as a starting material, with pyrrolidine nucleus as source of one of the two rings on which the second ring has been constructed.
CONCLUSIONS:
This review discusses the isolation of the deceptively simple pyrrolizidine alkaloid pyrrolam A, its biological studies, synthesis, and computational studies on the stability of the double bond in its strained bicyclic skeleton. In addition, the synthesis of pyrrolams B-C and their relationship to pyrrolam A is also discussed.