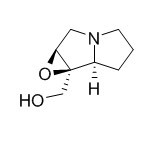
1,2-Epoxy-1-hydroxymethylpyrrolizidine
Reference standards.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Front Pharmacol.2022, 13:919230.
Oxid Med Cell Longev.2022, 2022:5888636.
Reprod Toxicol.2020, 96:1-10.
Nutrients.2023, 15(3):753.
Journal of Research in Pharmacy.2022, 26(6):p1752-1757.
Food Chem X.2024, 21:101127.
Cell Chem Biol.2019, 26(1):27-34
Sci Rep. 2018, 1-9
Asian Pac J Tropical Bio.2020, 10(6):239-247
Sci. Rep.2015, 14-23
Related and Featured Products
Plant Physiol. 1987 May;84(1):42-6.
Evidence for arginine as the endogenous precursor of necines in heliotropium.[Pubmed:
16665402]
METHODS AND RESULTS:
In pyrrolizidine alkaloid-bearing Heliotropium angiospermum and H. indicum shoots exposed, in the light, to (14)C-labeled CO(2) for 44 hours, the incorporation of (14)C into
1,2-Epoxy-1-hydroxymethylpyrrolizidine and retronecine amounted to 0.23 and 0.15%, respectively, of the total carbon assimilated. Treatment of the shoots with alpha-dl-difluoromethylornithine, the specific ornithine decarboxylase inhibitor, at 1 to 2 millimolar had no effect on (14)C incorporation into the necines. In contrast, alpha-dl-difluoromethylarginine, the specific arginine decarboxylase inhibitor, prevented the incorporation of (14)C into the necines of both species; the inhibitor did not affect the absolute incorporation of (14)C from exogenous [1,4-(14)C] putrescine in either species.
CONCLUSIONS:
Thus, arginine is the only apparent endogenous precursor of the putrescine channeled into pyrrolizidines, at least in these two Heliotropium species that exhibited a relatively much higher in vitro activity of arginine decarboxylase than of ornithine decarboxylase. However, within 28 hours after administration, not only exogenous l-[5-(14)C]arginine, but also exogenous l-[5-(14)C]ornithine exhibited significant incorporation of their label into the necines, incorporation that could be partially prevented by both inhibitors. Neither inhibitor affected the rates of (14)C-labeled CO(2) assimilation, transformation of labeled assimilates into ethanol-insoluble compounds, or the very high degree of conversion of the introduced amino acids into other compounds. Methodology related to alkaloid biosynthetic studies is discussed.