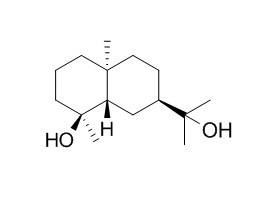
Pterodondiol
Pterodondiol has moderate activity against bacteria including Staphylococcus aureus, Pseudomonas aeruginosa, Bacillus subtilis, Mycobacteium phlei and Bacillus circulans.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Sci Rep.2019, 9(1):4646
Environ Toxicol.2023, 38(5):1174-1184.
J Ethnopharmacol.2023, 317:116789.
Eur J Pharmacol.2024, 981:176883.
Molecules.2022, 27(22):7997.
BMB Rep.2018, 51(5):249-254
J Korean Med Ophthalmol Otolaryngol Dermatol2023, 36(1):1-20.
Food Funct.2024, 15(4):1852-1866.
Acta Biochim Pol.2015, 62(2):253-8
Food Engineering Progress2019, 23(3)209-216
Related and Featured Products
Molecules. 2012 Dec 21;18(1):128-39.
Cytotoxic and antioxidant compounds from the stem bark of Goniothalamus tapisoides Mat Salleh.[Pubmed:
23344192]
METHODS AND RESULTS:
Eleven compounds:goniomicin A (1), goniomicin B (2), goniomicin C (3), goniomicin D (4), tapisoidin (5), goniothalamin (6), 9-deoxygoniopypyrone (7), Pterodondiol (8), liriodenine (9), benzamide (10) and cinnamic acid (11), were isolated from the stem bark of Goniothalamus tapisoides. All compounds were identified by spectroscopic analysis and, for known compounds, by comparison with published data.
CONCLUSIONS:
Goniothalamin (6) exhibited mild cytotoxic activity towards a colon cancer cell line (HT-29), with an IC(50)value of 64.17 ± 5.60 µM. Goniomicin B (2) give the highest antioxidant activity in the DPPH assay among all compounds tested, with an IC(50) of 0.207 µM.
Yao Xue Xue Bao. 2007 May;42(5):511-5.
Terpenoids and flavonoids from Laggera pterodonta.[Pubmed:
17703774]
METHODS AND RESULTS:
To study the chemical constituents of aerial parts of Laggera pterodonta (DC.) Benth., the air-dried aerial parts of this plant were powered and extracted with boiling water and purified by silica gel column chromatography and recrystallized. Eleven compounds were obtained from L. pterodonta. They were identified as to be 6-O-beta-D-glucopyranosyl-carvotanacetone (1), pterodontic acid (2), 1beta-hydroxy pterondontic acid (3), pterodontoside A (4), Pterodondiol (5), pterodontriol B (6), 5-hydroxy-3,4', 6,7-tetramethoxyflavone (7), artemitin (8), chrysosplenetin B (9), quercetin (10) and beta-sitosterol (11). Compound 1 is a new monoterpene glucoside. Compounds 10 and 11 were isolated from this plant for the first time.
CONCLUSIONS:
Compounds 2 and 5 showed moderate activity against bacteria including Staphylococcus aureus, Pseudomonas aeruginosa, Bacillus subtilis, Mycobacteium phlei and Bacillus circulans by paper disc diffusion method, while they both displayed no activity against Escherichia coli.