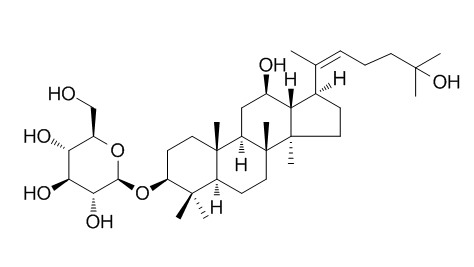
Pseudoginsenoside Rh2
Pseudoginsenoside Rh2 has cytotoxicity, it induces mitochondrial apoptosis in A549 cells and is responsible for excessive activation of the Ras/Raf/ERK/p53 pathway. (20Z) -Pseudoginsenoside Rh2 and (20E)-Pseudoginsenoside Rh2 have antioxidative activity.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Biomed Pharmacother.2024, 173:116319.
Int Immunopharmacol.2024, 141:112906.
Nutr Cancer.2024, 76(3):305-315.
Natural Product Communications2023, 18(9).
Pharmacogn Mag.2015, 11(43):562-6
J Applied Biological Chemistry2021, 64(2):185-192
Evid Based Complement Alternat Med.2021, 2021:8707280.
J Mater Chem B.2019, 7(39):5896-5919
Int J Mol Sci.2024, 25(5):2799.
Invest New Drugs.2017, 35(2):166-179
Related and Featured Products
Exp Ther Med. 2018 Jun;15(6):4916-4924.
Pseudo-Ginsenoside Rh2 induces A549 cells apoptosis via the Ras/Raf/ERK/p53 pathway.[Pubmed:
29805515]
Ginsenoside Rh2, a major effective constituent of ginseng, has been suggested to have a pro-apoptotic effect in a variety of cancer cells. Pseudoginsenside Rh2 (pseudo-G-Rh2) is a novel derivative of ginsenoside Rh2. The aim of the present study was to evaluate the effect of pseudo-G-Rh2 on the apoptosis of lung adenocarcinoma A549 cells.
METHODS AND RESULTS:
The cytotoxicity of pseudo-G-Rh2 on A549 cells was evaluated using an MTT assay. Apoptosis was detected using DAPI staining and flow cytometry. The expression of apoptosis associated proteins was identified by western blot analysis. The results demonstrated that pseudo-G-Rh2 inhibits the proliferation of A549 cells in a dose-dependent manner. DAPI staining revealed topical morphological changes in apoptotic bodies following pseudo-G-Rh2 treatment. Flow cytometric analysis revealed that the percentage of Annexin V-fluorescein isothiocyanate-positive cells, which are apoptotic, increased with pseudo-G-Rh2 treatment in a dose-dependent manner. Furthermore, treatment with pseudo-G-Rh2 increased the level of reactive oxygen species in A549 cells as well as the activation of caspase-9, caspase-3 and poly ADP-ribose polymerase. Pseudo-G-Rh2 treatment was observed to induce mitochondrial membrane potential loss. Furthermore, the results of western blotting revealed that B-cell lymphoma 2 (Bcl-2) expression was significantly decreased while Bcl-2-associated X protein expression was significantly upregulated in A549 cells with pseudo-G-Rh2 treatment. Pseudo-G-Rh2-induced apoptosis was accompanied by sustained phosphorylation of Ras, Raf, extracellular signal-regulated kinase (ERK) and p53.
CONCLUSIONS:
In conclusion, the results of the present study suggest that pseudo-G-Rh2 induces mitochondrial apoptosis in A549 cells and is responsible for excessive activation of the Ras/Raf/ERK/p53 pathway.
Chem Pharm Bull (Tokyo). 2018 May 1;66(5):535-540.
Semi-synthesis of Twelve Known 20Z/E Pseudo-Ginsenosides and Their Comparative Study of Antioxidative Activity in Free Radical Induced Hemolysis of Rabbit Erythrocytes.[Pubmed:
29515052]
METHODS AND RESULTS:
Twelve pseudo-ginsenosides were synthesized under a mild condition, via a simple three-step called acetylation, elimination-addition and saponification. The inhibitory effects of these twelve pseudo-ginsenosides were screened on the hemolysis of rabbit erythrocytes caused by 2,2'-azobis (2-amidinopropane hydrochloride) (AAPH).It was found that the IC50 values followed the sequence of (20Z) pseudo-protopanaxatriol (pseudo-PPT)<(20Z) pseudo-protopanaxadiol (pseudo-PPD)<(20Z) pseudo-Rh2(Pseudoginsenoside Rh2)<(20E) pseudo-PPT<(20E) pseudo-PPD<(20E) pseudo-Rh2(Pseudoginsenoside Rh2)<(20Z) pseudo-Rg2<(20E) pseudo-Rg2 CONCLUSIONS:
These compounds can be divided into three groups: accelerate the hemolysis group (7, 8), weak group (2, 11, 12) and strong group (others). Moreover, we also find that most of the Z configuration has better antioxidative activity than E configuration and the number and type of sugar moieties to the ring of triterpene dammarane influence the antioxidative activity.