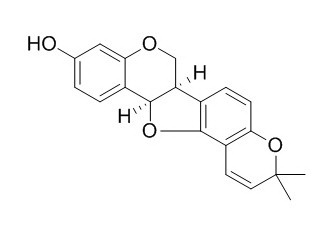
Phaseollin
Phaseollin and neorautenol may be responsible for the anticarcinogenic actions of the plant extract, may lead to new pharmacons to be used in cancer therapy.9-Aminoacridine and other DNA intercalators function as inducers of Phaseollin and phenylalanine ammonia lyase synthesis by reacting with the DNA template.The red kidney bean phytoalexins kievitone and phaseollin possess both estrogenic and antiestrogenic activities.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Turkish Journal of Pharmaceutical Sciences2022, DOI: 10.4274
Plant Foods Hum Nutr.2021, 76(4):472-477.
Phytomedicine.2023, 117:154929.
The Journal of Supercritical Fluids2021, 176:105305.
Ind. J. Pharm. Edu. Res.2023; 57(3):1132-1139.
Antioxidants (Basel).2023, 12(1):189.
Asian Pac J Cancer Prev.2019, 20(1):65-72
Anal Chim Acta.2018, 1039:162-171
Pharmaceuticals (Basel).2024, 17(3):352.
Molecules.2024, 29(5):1050.
Related and Featured Products
J Agric Food Chem. 2011 Jan 12;59(1):112-20.
Estrogenic and antiestrogenic activities of phytoalexins from red kidney bean (Phaseolus vulgaris L.).[Pubmed:
21133423 ]
METHODS AND RESULTS:
The phytoalexins kievitone and Phaseollin were isolated from A. sojae-treated red kidney bean extracts and analyzed for estrogenic activity using ERα and ERβ binding, ERE luciferase assays in MCF-7 and HEK 293 cells, and MCF-7 cell proliferation. Kievitone showed the highest relative binding affinity to ERα with kievitone (0.48%) > Phaseollin (0.21%), and Phaseollin showed the highest relative binding affinity to ERβ with Phaseollin (0.53%) > kievitone (0.42%). In an ERE luciferase assay in MCF-7 cells, kievitone displayed high ER transactivation at 10 μM; Phaseollin displayed low ER transactivation. Both kievitone and Phaseollin stimulated MCF-7 cell proliferation, with kievitone displaying agonist activity between 0.1 and 10 μM. Cotransfection reporter assays performed in HEK 293 demonstrated that Phaseollin selectively increased ERE transcriptional activity of ERβ and kievitone selectively increased ERE transcriptional activity of ERα. Although Phaseollin displayed attenuation of ER transactivation in the ERE luciferase assay in MCF-7 cells, both phytoalexins attenuated the effects of E2 in an MCF-7 cell colonial survival assay.
CONCLUSIONS:
This work provides evidence that the red kidney bean phytoalexins kievitone and Phaseollin possess both estrogenic and antiestrogenic activities.
Toxicology. 2007 Dec 5;242(1-3):71-9.
Pterocarpans phaseollin and neorautenol isolated from Erythrina addisoniae induce apoptotic cell death accompanied by inhibition of ERK phosphorylation.[Pubmed:
17964704]
The genus Erythrina (Leguminosae), consisting of over 100 different species, is distributed in tropical regions. In traditional medicine, Erythrina species are used to treat cancer, but little is known about the anticancer mechanisms.
METHODS AND RESULTS:
From the stem bark of Erythrina addisoniae Hutch. & Dalziel, six prenylated pterocarpans were isolated and analysed for pharmacological activity: While calopocarpin, cristacarpin, orientanol c, and isoneorautenol showed only a weak or moderate toxicity in H4IIE hepatoma cells (EC(50)-value> 25 microM), the toxicity of neorautenol and Phaseollin was in the low micromolar range (EC(50)-value: 1 and 1.5 microM, respectively). We further focused on these two substances showing that both increased caspase 3/7 activity and nuclear fragmentation as markers for apoptotic cell death. Neorautenol (10 microM, 2h), but not Phaseollin induced the formation of DNA strand breaks (comet assay). Both substances showed no effect on NF-kappaB signalling (SEAP assay: basal activity and stimulation with TNF-alpha), on the other hand both pterocarpans (10 microM, 2 h) decreased the activation of the ERK kinase (p44/p42), an mitogen activated protein kinase which is associated with cell proliferation.
CONCLUSIONS:
We conclude that the pterocarpans Phaseollin and neorautenol may be responsible for the anticarcinogenic actions of the plant extract reported in the literature. Further analysis of these substances may lead to new pharmacons to be used in cancer therapy.
Plant Physiol. 1971 Aug;48(2):197-202.
The induction of phenylalanine ammonia lyase and phaseollin by 9-aminoacridine and other deoxyribonucleic Acid intercalating compounds.[Pubmed:
16657762]
METHODS AND RESULTS:
Bean pod tissue (Phaseolus vulgaris L. var. Top Crop) is induced to produce Phaseollin when challenged with various microorganisms.
The pods react in the same manner when challenged with 9-aminoacridine. This compound also caused an increase in concentrations of phenylalanine ammonia lyase, an enzyme of the Phaseollin synthesizing pathway.
Both the synthesis of phenylalanine ammonia lyase and Phaseollin are subject to inhibition by actinomycin D, cycloheximide, or 6-methylpurine. The results suggest that both Phaseollin production and increased phenylalanine ammonia lyase, when induced by 9-aminoacridine, require newly synthesized RNA and protein.The concentration of 9-aminoacridine optimal for synthesis of Phaseollin and PAL (0.5 mg/ml) does not increase the rate of total protein synthesis. However, there is a differential effect of 9-aminoacridine on synthesis of certain protein fractions. Optimal concentrations of 9-aminoacridine induce Phaseollin and phenylalanine ammonia lyase synthesis while reducing the net synthesis of RNA during the period of induction.The planar three-ring structure of 9-aminoacridine appears to be a desirable feature for Phaseollin and phenylalanine ammonia lyase induction. Similar compounds, all DNA intercalators, having dimethylamino, diethylamino, amino, or 9-alkylamino substitutions of a three-ring acridine skeleton, are also inducers of phenylalanine ammonia lyase and Phaseollin synthesis.
CONCLUSIONS:
It is suggested that 9-aminoacridine and other DNA intercalators function as inducers of Phaseollin and phenylalanine ammonia lyase synthesis by reacting with the DNA template.