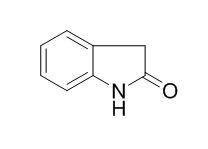
Oxindole
Oxindole structure has been used in receptor tyrosine kinases (RTKs) inhibitors such as SU4984 and intedanib, the RTK family represents an important therapeutic target for anti-cancer drug development.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Journal of Ginseng Research2023, 12.004.
Nutrients.2024, 16(14):2267.
Nutrients.2019, 11(11):E2694
J Health Sci Med Res.2023, 31584.
Life Sci.2021, 286:120019.
Agriculture2024, 14(12), 2301
Toxins (Basel).2021, 13(12):898.
J AOAC Int.2024, qsae028.
Evid Based Complement Alternat Med.2017, 2017:6360836
Toxicol In Vitro.2023, 93:105667.
Related and Featured Products
Bioorg Med Chem Lett. 2015 Aug 15;25(16):3285-9.
Synthesis of novel derivatives of oxindole, their urease inhibition and molecular docking studies.[Pubmed:
26077497]
We synthesized a series of novel 5-24 derivatives of Oxindole.
METHODS AND RESULTS:
The synthesis started from 5-chloroOxindole, which was condensed with methyl 4-carboxybezoate and result in the formation of benzolyester derivatives of Oxindole which was then treated with hydrazine hydrate. The Oxindole benzoylhydrazide was treated with aryl acetophenones and aldehydes to get target compounds 5-24. The synthesized compounds were evaluated for urease inhibition; the compound 5 (IC50 = 13.00 ± 0.35 μM) and 11 (IC50 = 19.20 ± 0.50 μM) showed potent activity as compared to the standard drug thiourea (IC50 = 21.00 ± 0.01 μM). Other compounds showed moderate to weak activity.
CONCLUSIONS:
All synthetic compounds were characterized by different spectroscopic techniques including (1)H NMR, (13)C NMR, IR and EI MS.The molecular interactions of the active compounds within the binding site of urease enzyme were studied through molecular docking simulations.
Bioorg Med Chem. 2014 Dec 15;22(24):6953-60.
Synthesis and biological evaluation of novel oxindole-based RTK inhibitors as anti-cancer agents.[Pubmed:
25456085]
Given that receptor tyrosine kinases (RTKs) have emerged as key regulators of all aspects of cancer development, including proliferation, invasion, angiogenesis and metastasis, the RTK family represents an important therapeutic target for anti-cancer drug development. Oxindole structure has been used in RTK inhibitors such as SU4984 and intedanib.
METHODS AND RESULTS:
In this study, two series of new heterocyclic compounds containing Oxindole scaffold have been designed and synthesized, and their inhibitory activity against the proliferation of nine cancer cell lines has been evaluated. Among them, compounds 9a and 9b displayed the strongest anti-proliferative activity with the IC50s below 10μM. Flow cytometric analysis showed that the compounds 9a and 9b dose-dependently arrested the cell cycle at G0/G1 phase. Although the leading compounds SU4984 and intedanib targets FGFR1, the kinase activity test revealed that these compounds only showed slight inhibitory activity on FGFR1 kinase.
CONCLUSIONS:
Further enzymatic test aided by molecular docking simulation in the ATP-binding site demonstrated that 9a and 9b are potent inhibitors of c-Kit kinase. These compounds are worthy of further evaluation as anticancer agents.
Epimedin K
Catalog No: CFN95019
CAS No: 174286-13-6
Price: $288/5mg
7,2',4'-Trihydroxy-5-methoxy-3-arylcoumarin
Catalog No: CFN95070
CAS No: 1092952-62-9
Price: Inquiry(manager@chemfaces.com)
Caraganaphenol A
Catalog No: CFN95093
CAS No: 174916-31-5
Price: $333/5mg
(1,5E,11E)-tridecatriene-7,9-diyne-3,4-diacetate
Catalog No: CFN95161
CAS No: 201012-14-8
Price: $318/10mg
4,4-di(4-hydroxy-3-methoxyphenly)-2,3-dimethylbutanol
Catalog No: CFN95200
CAS No: 913643-31-9
Price: $368/5mg
Rebaudioside F
Catalog No: CFN95215
CAS No: 438045-89-7
Price: $268/5mg
6-Hydroxy-7-methoxydihydroligustilide
Catalog No: CFN95289
CAS No: 210036-09-2
Price: $318/5mg
3',4',5',5,7-Pentamethoxyflavanone
Catalog No: CFN95414
CAS No: 479672-30-5
Price: $218/20mg
Campneoside II
Catalog No: CFN95498
CAS No: 95587-86-3
Price: $318/10mg
Scoparin
Catalog No: CFN95544
CAS No: 301-16-6
Price: $318/5mg