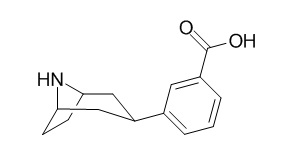
Nortropacocaine
Reference standards.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Food Chem.2017, 228:301-314
Environ Toxicol.2024, 39(5):2927-2936.
Phytomedicine.2022, 102:154183.
Biosci Biotechnol Biochem.2020, 84(3):621-632
Ind. J. Pharm. Edu. Res.2023; 57(3):1132-1139.
Nat Prod Commun.2018, 10.1177
Eur J Pharmacol.2023, 960:176121.
Biomed Pharmacother.2022, 146:112497.
Nutr Cancer.2022, 1-13.
Food Bioscience2024, 58:103691.
Related and Featured Products
J Pharm Sci. 1979 Jun;68(6):788-90.
Nortropacocaine hydrochloride conformation in aqueous and hydrophobic media.[Pubmed:
458585]
The Nortropacocaine hydrochloride PMR spectra in deuterium oxide and in deuterochloroform differed markedly.
METHODS AND RESULTS:
A detailed conformational analysis using vicinal 1H-1H coupling constants revealed the molecular conformation to be identical in both solvents. The preferred conformation was one in which the piperidine component existed as a deformed chair.
The spectral differences were due to a decreased deshielding of the protonated nitrogen on the neighboring bicyclic ring protons, resulting in chemical shift changes.
Kakkalide
Catalog No: CFN95052
CAS No: 58274-56-9
Price: $260/10mg
Lucidumol A
Catalog No: CFN95059
CAS No: 217476-73-8
Price: $318/5mg
5,7,2',4'-Tetrahydroxy-8,3'-di(gamma,gamma-dimethylallyl)-isoflavanone
Catalog No: CFN95084
CAS No: 141846-47-1
Price: $413/5mg
(1,5E,11E)-tridecatriene-7,9-diyne-3,4-diacetate
Catalog No: CFN95161
CAS No: 201012-14-8
Price: $318/10mg
Licoricesaponin H2
Catalog No: CFN95173
CAS No: 118441-85-3
Price: $368/20mg
Platycogenin A
Catalog No: CFN95240
CAS No: 1459719-53-9
Price: $388/5mg
Cuneataside C
Catalog No: CFN95368
CAS No: 871720-16-0
Price: $318/10mg
4'-Hydroxy-3',5,5',6,7,8-hexamethoxyflavone
Catalog No: CFN95407
CAS No: 85644-03-7
Price: $318/5mg
Malabaricone A
Catalog No: CFN95477
CAS No: 63335-23-9
Price: $318/10mg
Oxytroflavoside B
Catalog No: CFN95490
CAS No: 1391144-81-2
Price: $318/10mg