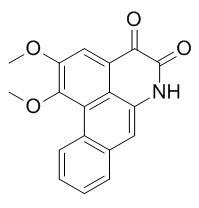
Norcepharadione B
Norcepharadione B shows good inhibitory activity against the replication of HSV-1, it also shows antimalarial activity with EC50 values of 7.5mug/ml. Norcepharadione B exhibits significant cytotoxicity against five human tumor cell lines (A-549, SK-OV-3, SK-MEL-2, XF-498 and HCT-15) in vitro. Norcepharadione B shows significant inhibitory effects on both ADP-induced and thrombin-induced platelet aggregation.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Bioorg Chem.2024, 150:107558.
Anal Bioanal Chem. 2016, 408(15)
Front Microbiol.2022, 12:833233.
Nutrients.2021, 13(1):254.
J Food Sci Technol.2019, 56(5):2712-2720
Phytomedicine.2024, 122:155065.
Plant Cell Physiol.2018, 59(1):128-141
Plants (Basel).2023, 12(5):1120.
Plant Foods Hum Nutr.2020, 10.1007
Journal of Mushroom2023, 21(4):215-221.
Related and Featured Products
Chem Pharm Bull (Tokyo). 2009 Nov;57(11):1227-30.
The constituents and their bioactivities of Houttuynia cordata.[Pubmed:
19881272]
METHODS AND RESULTS:
Chemical investigation on the whole plant of Houttuynia cordata has resulted in the isolation of two new compounds, named as houttuynoside A (1) and houttuynamide A (2), together with thirty-eight known compounds. The structures of 1 and 2 were elucidated on the basis of spectroscopic analysis. In the inhibitory effects on herpes simplex virus type 1 (HSV-1) assay, Norcepharadione B (10) showed good inhibitory activity against the replication of HSV-1. In addition, the antioxidant and antityrosinase activities of some isolated compounds were also evaluated.
CONCLUSIONS:
Among these compounds, quercitrin (25) and quercetin-3-O-beta-D-galactopyranoside (26) showed excellent 2,2-diphenyl-1-picrylhydrazyl radical-scavenging property with IC50 values of 31 and 63 microM, respectively. Cepharadione B (9) exhibited strong tyrosinase inhibitory activity with an IC50 value of 170 microM.
Arch Pharm Res. 2001 Dec;24(6):518-21.
Cytotoxic alkaloids from Houttuynia cordata.[Pubmed:
11794526]
METHODS AND RESULTS:
Six bioactive alkaloids, aristolactam B(1), piperolactam A(2), aristolactam A(3), Norcepharadione B(4), cepharadione B(5) and splendidine(6) were isolated by bioactivity-guided fractionation of a methanolic extract of the aerial part of Houttuynia cordata.
CONCLUSIONS:
Several of them exhibited significant cytotoxicity against five human tumor cell lines (A-549, SK-OV-3, SK-MEL-2, XF-498 and HCT-15) in vitro.
Chinese Journal of Natural Medicines, 2011, 9(6):425-8.
Alkaloids from Houttuynia cordata and Their Antiplatelet Aggregation Activities.[Reference:
WebLink]
To study the chemical constituents from Houttuynia cordata and to test their antiplatelet aggregation activities.Methods The chemical constituents were isolated and purified by silica gel column chromatography and their structures were elucidated on the basis of spectral analysis. The antiplatelet aggregation activities were evaluated by Born's method, using rat PRP induced by ADP and thrombin.ResultsNine alkaloids were isolated and their structures were identified as Norcepharadione B (1), 4, 5-dioxodehydroasimilobine (2), cepharadione B (3), aristololactam B II (4), aristololactam A II (5), sauristolactam (6), piperolactam A (7), splendidine (8), and aristololactam F II (9). Antiplatelet aggregation test indicated significant inhibitory activities of compounds 1, 2, 4–7, 9 induced by ADP and 1, 2, 4, 7 induced by thrombin.Conclusion Compounds 2, 6, 7, 9 were isolated from the genus Houttuynia for the first time. Compounds 1, 2, 4, 7 showed significant inhibitory effects on both ADP-induced and thrombin-induced platelet aggregation.
Chulalongkorn University. 1996.
Antimalarial compounds from Goniothalamus tenuifolius.[Reference:
WebLink]
METHODS AND RESULTS:
1996 In the search for antimalarial compounds from Goniothalamus tenuifolius, four aristolactam alkaloids, namely aristolactam BI, aristolactam BII, velutinam and aristolactam AII were isolated along with a 4, 5-dioxoaporphine alkaloid named Norcepharadione B, and an ethyl ester of 2, 4-dihydroxy-6-methylbenzoic acid. The structure identifications of all of the isolated compounds, including their unequivocal 13C NMR assignments, were achieved by analysis of their UV, IR, MS and NMR data. All compounds were evaluated for their antimalarial activity against Plasmodium falciparum T9/94 by radioisotope microdilution technique.
CONCLUSIONS:
The ethyl ester of 2, 4-dihydroxy-6-methylbenzoic acid, aristolactam BI, aristolactam BII, velutinam, Norcepharadione B and aristolactam AII showed EC50 values of 33, 10.5, 11, 7.5, 7.5 and 9.5 mug/ml, respectively, whereas those of chloroquine and pyrimethamine were 0.03 and 2.8 mug/ml, respectively. This investigation is the first report of antimalarial activity of aristolactam alkaloids.