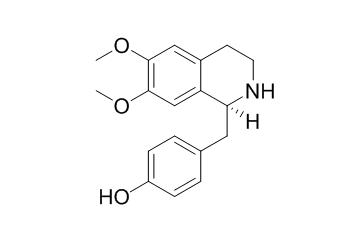
Norarmepavine
N-norarmepavine shows significant cytotoxic activities against HL-60 carcinoma cell line with inhibitory ratios of 51.43% at concentration of 1 x 10(-5) M. Norarmepavine shows inhibition against Trypanosoma cruzi. D-(+)- N-norarmepavine exhibits significant inhibitory activity towards adenosine 5'-diphosphate (ADP)-, arachidonic acid (AA)-, collagen-, and/or platelet-activating factor (PAF)-induced platelet aggregation.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Front Immunol.2017, 8:1542
J Med Food.2016, 19(12):1155-1165
Appl. Sci. 2021, 11(22),10569
Front Cell Infect Microbiol.2018, 8:292
Korean Herb. Med. Inf. 2016, 4(1):35-42
Phytomedicine.2022, 100:154036.
bioRxiv2021, 458409.
The Japan Society for Analy. Chem.2017, 66(8):613-617
Plant Physiol Biochem.2023, 203:108073.
LWT2020, 124:109163
Related and Featured Products
Zhongguo Zhong Yao Za Zhi. 2013 Dec;38(23):4104-8.
Cytotoxic alkaloids from stems of Nelumbo nucifera.[Pubmed:
24791498]
Chemical investigation was carried out to study the alkaloids from stems of Nelumbo nucifera and their cytotoxic activities.
METHODS AND RESULTS:
The constituents were separated by column chromatography, and their structures were elucidated by spectroscopic data analyses. The isolated compounds were evaluated for their cytotoxic activities by MTr method. Fifteen compounds were isolated from the total alkaloids extract and identified as asimilobine (1), isococlaurine (2), N-acetylNorarmepavine (3), crykonisine (4), velucryptine (5), pycnarrhine (6), liriodenine (7), nuciferine (8), nornuciferine (9), armepavine (10), N-methylasimilobine (11), coclaurine (12), N-Norarmepavine (13), N-methylcoclaurine (14) and lysicamine (15). Compounds 1-7 and 12-15 were isolated from stems of this plant for the first time, and compounds 2-6 were firstly isolated from the family Nelumbonaceae.
CONCLUSIONS:
Compounds 7-10, 13 and 14 showed significant cytotoxic activities against HL-60 carcinoma cell line with inhibitory ratios of 51.36%, 59.09%, 52.51%, 53.93%, 51.43%, and 64.31% at concentration of 1 x 10(-5) mol x L(-1), respectively.
Planta Med. 2006 Oct;72(13):1238-41.
Effect of isoquinoline alkaloids of different structural types on antiplatelet aggregation in vitro.[Pubmed:
16981134 ]
Forty-one isoquinoline alkaloids were tested for antiplatelet aggregation effects. Among them, (-)-discretamine (6), protopine (7), ochotensimine (18), O-methylarmepavinemethine (23), lindoldhamine (25), isotetrandrine (26), thalicarpine (27), papaverine (28), and D-(+)- N-Norarmepavine (32) exhibited significant inhibitory activity towards adenosine 5'-diphosphate (ADP)-, arachidonic acid (AA)-, collagen-, and/or platelet-activating factor (PAF)-induced platelet aggregation. The results are discussed on the basis of structure-activity relationships.
Comp Biochem Physiol Pharmacol Toxicol Endocrinol. 1994 Mar;107(3):367-71.
Trypanocidal effect of boldine and related alkaloids upon several strains of Trypanosoma cruzi.[Pubmed:
8061943]
METHODS AND RESULTS:
The alkaloids boldine, glaucine, predicentrine, apomorphine, coclaurine, Norarmepavine and codeine were tested against the epimastigotes of the Tulahuén and LQ strains and the DM 28c clone of Trypanosoma cruzi. The micromolar concentration to inhibit 50% of the culture growth (Tulahuén strain) for apomorphine, glaucine, predicentrine, boldine, Norarmepavine, coclaurine and codeine were 29, 90, 85, 110, 310, 580 and > 1000 respectively. Similar values were obtained with the LQ strain and the DM 28c clone.
CONCLUSIONS:
The most active compounds in inhibiting culture growth also inhibited cell respiration, suggesting that these drugs may act by blocking mitochondrial electron transport. The trypanocidal effects of these alkaloids appear to be correlated with their antioxidative activities.