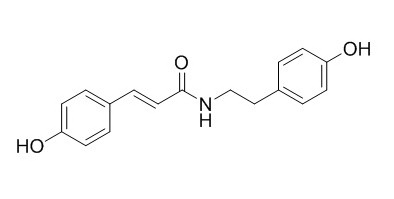
N-p-trans-Coumaroyltyramine
N-p-trans-Coumaroyltyramine is an inhibitor on acetylcholinesterase (AChE), it inhibits AChE activity in a dose-dependent manner with IC50 value of 34.5 microg/mL (122 microM). It exhibits potent inhibition of cell proliferation, platelet aggregation, and shows antioxidant activity. N-trans-p-coumaroyltyramine shows activity against Trypanosoma brucei rhodesiense (IC50s ranging from 2.2 to 13.3 microM).
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
J Pharmacopuncture.2023, 26(4):357-365.
Chemistry of Plant Materials.2019, 215-222
Eur J Pharmacol.2023, 950:175772.
J Biochem Mol Toxicol.2021, 35(5):e22731.
Evid Based Complement Alternat Med.2021, 2021:5585692.
J Nat Sc Biol Med2019, 10(2):149-156
Journal of Functional Foods2021, 84:104581
J Food Composition and Analysis2022, 104417.
Drug Des Devel Ther.2020, 14:61-71
Nutrients.2020, 12(5):1242.
Related and Featured Products
Arch. Pharm. Res.,2003, 26(9):735-8.
Inhibitory effect of trans-N-p-coumaroyl tryamine from the twigs of Celtis chinensis on the acetylcholinesterase.[Pubmed:
14560923]
METHODS AND RESULTS:
The methanolic extract of the twigs of Celtis chinensis was found to show inhibitory activity on acetylcholinesterase (AChE), an enzyme that plays a role in the metabolic hydrolysis of ACh. Bioassay-guided fractionation of the methanolic extract resulted in the isolation of N-p-trans-Coumaroyltyramine, as an inhibitor on AChE.
CONCLUSIONS:
This compound inhibited AChE activity in a dose-dependent manner, and the IC50 value of N-p-trans-Coumaroyltyramine was 34.5 microg/mL (122 microM).
Nat Prod Commun. 2012 Jun;7(6):753-5.
Cinnamoylphenethyl amides from Polygonum hyrcanicum possess anti-trypanosomal activity.[Pubmed:
22816300]
METHODS AND RESULTS:
A methanolic extract from aerial parts of Polygonum hyrcanicum (Polygonaceae) showed high activity against Trypanosoma brucei rhodesiense (IC50 = 3.7 microg/mL). Bioassay-guided fractionation of the extract resulted in isolation of cinnamoylphenethyl amides, including N-trans-caffeoyltyramine (1), N-p-trans-Coumaroyltyramine (7), and N-trans-feruloyltyramine (8) as the main active constituents (IC50s ranging from 2.2 to 13.3 microM). Some structurally related, but less active compounds, such as cannabisin B (2), tyrosol (3), p-coumaric acid (4), ferulic acid (5), and N-cis-feruloyltyramine (6) were also identified, along with N-trans-3,4-dimethoxycinnamoyldopamine (9).
CONCLUSIONS:
Cytotoxicity of the active compounds in L6 cells was determined, and selectivity indices (SI) of 7.9 to 33.4 were calculated.
Bioscience Biotechnology & Biochemistry, 2014, 61(7):1138-1141.
Isolation and Activity of N-p-Coumaroyltyramine, an α-Glucosidase Inhibitor in Welsh Onion (Allium fistulosum)[Reference:
WebLink]
A phenolic amide, N-p-trans-Coumaroyltyramine(1), was isolated as an α-glucosidase inhibitor from methanol extracts of Welsh onion (Allium fistulosum). The inhibitory activity of 1 against a yeast enzyme was as high as Ki 8.4 × 10617 m. From a structure-activity relationship study of 1 and its related compounds, the occurrence of α-glucosidase inhibitory activity required a p-coumaramide structure, with an amide hydrogen and alkyl or aralkyl substituent on the amide part.
Nat Prod Commun. 2011 Feb;6(2):227-9.
Amides from the stem of Capsicum annuum.[Pubmed:
21425680]
METHODS AND RESULTS:
7'-(4'-hydroxyphenyl)-N-[(4-methoxyphenyl)ethyl]propenamide (1), 7'-(3',4'-dihydroxyphenyl)-N-[(4-methoxyphenyl)ethyl]propenamide (2), N-p-trans-Coumaroyltyramine (3), N-trans-caffeoyltyramine (4), beta-sitostenone (5), ferulic acid (6), hydroferulic acid (7), 5-hydroxy-3,4-dimethoxycinnamic acid (8), veratic acid (9), vanillic acid (10), isovanillic acid (11), syringic acid (12), (+)-syringaresinol (13), and pheophorbide a (14) were isolated from the stems of Capsicum annuum (Solanaceae). Among them, 1 is a new amide compound.
CONCLUSIONS:
The structures of these compounds were characterized and identified by spectral analyses.
J. Phys. Chem. B, 2010, 114(8):3005-12.
Interaction Studies of Coumaroyltyramine with Human Serum Albumin and Its Biological Importance[Pubmed:
20136105]
N-trans-p-coumaroyltyramine (N-p-trans-Coumaroyltyramine, CT) isolated from Physalis minima is a phenolic substance exhibiting many pharmacological activities like potent inhibition of acetyl cholinesterase, cell proliferation, platelet aggregation, and also antioxidant activity.
METHODS AND RESULTS:
Here, we have studied the binding of CT with HSA at physiological pH 7.2 by using fluorescence, circular dichroism spectroscopy, mass spectrometry, and molecular docking methods. From the fluorescence emission studies, the number of binding sites and binding constant were calculated to be 2 and (4.5 +/- 0.01) x 10(5) M(-1), respectively. The free energy change was calculated as -7.6 kcal M(-1) at 25 degrees C, which indicates the hydrophobic interactions of CT with HSA and is in well agreement with the computational calculations and molecular docking studies. The changes in the secondary structure of HSA after its complexation with the ligand were studied with CD spectroscopy, which indicated that the protein became partially unfolded. Also, temperature did not affect the HSA-CT complexes.
CONCLUSIONS:
The binding of CT with HSA was detected as 2 molecules bound to HSA was determined using micro TOF-Q mass spectrometry. Further, molecular docking studies revealed that CT was binding at subdomain IIA with hydrophobic interactions and also by hydrogen-bond interactions between the hydroxyl (OH) group of carbon-16 and carbon-2 of CT and Arg222, Ala291, Val293, and Met298 of HSA, with hydrogen-bond distances of 2.488, 2.811, 2.678, and 2.586 A, respectively.