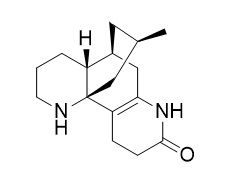
N-Demethyl-alpha-obscurine
N-Demethyl-alpha-obscurine is a natural product from Lycopodium japonicum.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Nat Plants.2016, 3:16205
Sci Rep.2021, 11(1):21038.
Molecules.2024, 29(23):5632.
J Ethnopharmacol.2018, 210:88-94
Nat Prod Commun.2017, 12(5):771-778
Nutrients.2024, 16(22):3805.
J Pharm Biomed Anal.2018, 151:32-41
Srinakharinwirot University2023, 2669.
Journal of Phytopathology2021, 169,Issue11-12.
ACS Synth Biol.2020, 9(9):2282-2290.
Related and Featured Products
Chinese Traditional & Herbal Drugs, 2015, 46(9):1269-1276.
Lycopodium alkaloids from Lycopodium japonicum.[Reference:
WebLink]
To study the chemical constituents in the whole herb of Lycopodium japonicum.
METHODS AND RESULTS:
The constituents were isolated and purified by silica gel and semi-preparative HPLC, and their structures were elucidated by means of physicochemical properties and spectroscopic analysis. All the isolated compounds were evaluated using relevant in vitro anti-inflammatory assay against LPS-induced NO releases.Nineteen compounds were isolated from the whole herb of L. japonicum, and identified as lycoposerramine-M N-oxide (1), acetyllycoposerramine-M (2), lycopodine (3), lycoposerramine-M (4), miyoshianine-C (5), 12-epilycodoline N-oxide (6), gnidioidine (7), lycoposerramine-K (8), lucidioline (9), 4α-hydroxyanhydrolycodoline (10), flabelline (11), hydroxypropyllycodine (12), lycodine (13), des-N-methyl-α-obscurine (N-Demethyl-alpha-obscurine,14), α-obscurine (15), des-N-methyl-β-obscurine (16), lycoflexine (17), lycoflexine N-oxide (18), and fawcettidine (19). Compound 5 and 18 could inhibit the release of nitric oxide (NO) in the RAW264.7 cell line stimulated by lipopolysaccaride. The IC50 values of 5 and 18 are 31.82 and 40.69 μmol/L, respectively.
CONCLUSIONS:
Compound 1 is a new compound, named lycoposerramine-M N-oxide, compounds 2, 6, 11, 12, 16, 18, and 19 are isolated from this plant for the first time. Compounds 5 and 18 exhibit the potent inhibitory activity.
European Journal of Organic Chemistry, 1983, 1983(2):220-225.
Synthese desLycopodium-Alkaloidsrac-α-Obscurin durch 1,3-Anellierung eines Enimins.[Reference:
WebLink]
METHODS AND RESULTS:
Synthesis of the Lycopodium-Alkaloids rac-α-Obscurin through 1,3-Anullation of an Enimine Acid catalysed 1,3-annulation of 1,2,3,4-tetrahydro-6-methyl-2-oxopyridine (1) to the enimine 3, followed by methylation, provides a new regio and stereoselective route to rac-α-obscurine (5). Reduction of rac-N-Demethyl-alpha-obscurine with LiAlH4 yields rac-lycodine (11) and rac-deacetyl-flabellidine (9).
CONCLUSIONS:
A new pathway for the biogenesis of the β-amino-imine 9 is suggested.