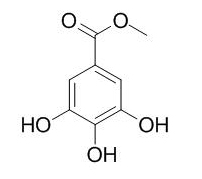
Methyl gallate
Methyl Gallate is a plant polyphenol with antioxidant, anticancer, anti-bacterial, and anti-inflammatory activities.Methyl gallate is a potent and highly specific inhibitor of herpes simplex virus in vitro. Methyl gallate also has a dual cyclooxygenase-2/5-lipoxygenase inhibitory activity. Methyl gallate can inhibit the growth of oral pathogens and S. mutans biofilm formation, and may be used to prevent the formation of oral biofilms.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
J Asian Nat Prod Res.2019, 5:1-17
Food Res Int.2024, 191:114613.
Anim Cells Syst (Seoul).2024, 28(1):381-391.
Ann Transl Med.2019, 7(23):731
Exp Mol Med.2020, 52(4):629-642.
BMC Complement Med Ther. 2020, 20(1):91.
ACS Synth Biol.2022, doi: 10.1021.
Environ Toxicol.2023, 38(5):1174-1184.
Int J Mol Sci.2017, 18(12)
Tumour Biol.2015, 36(12):9385-93
Related and Featured Products
J Microbiol. 2008 Dec;46(6):744-50.
Inhibitory effect of methyl gallate and gallic acid on oral bacteria.[Pubmed:
19107406 ]
This study examined the ability of Methyl gallate (MG) and gallic acid (GA), the main compounds of gallo-tannins in Galla Rhois, to inhibit the proliferation of oral bacterial and the in vitro formation of Streptococcus mutans biofilms.
METHODS AND RESULTS:
The antimicrobial activities of these compounds were evaluated in vitro using the broth microdilution method and a beaker-wire test. Both MG and GA had inhibitory effects on the growth of cariogenic (MIC<8 mg/ml) and periodontopathic bacteria (MIC=1 mg/ml). Moreover, these compounds significantly inhibited the in vitro formation of S. mutans biofilms (MG, 1 mg/ml; GA, 4 mg/ml; P<0.05). MG was more effective in inhibiting bacterial growth and the formation of S. mutans biofilm than GA.
CONCLUSIONS:
In conclusion, MG and GA can inhibit the growth of oral pathogens and S. mutans biofilm formation, and may be used to prevent the formation of oral biofilms.
Molecules. 2009 May 11;14(5):1773-80.
Antibacterial activity of methyl gallate isolated from Galla Rhois or carvacrol combined with nalidixic acid against nalidixic acid resistant bacteria.[Pubmed:
19471197]
Methyl gallate is a major component of Galla Rhois, as carvacrol is of oregano essential oils. Both have shown good antibacterial activity against intestinal bacteria.
METHODS AND RESULTS:
This study investigated the antibacterial activities of nalidixic acid in combination with Methyl gallate and carvacrol against nalidixic acid resistant bacteria. The combined effect of nalidixic acid with Methyl gallate and carvacrol was evaluated using the checkerboard method to obtain a fractional inhibitory concentration index. The results showed that the combinations of nalidixic acid + Methyl gallate/carvacrol improved nalidixic acid resistant pathogenic bacteria inhibition with synergy or partial synergy activity. Thus, a strong bactericidal effect of the drug combinations was observed.
CONCLUSIONS:
In vitro data thus suggested that nalidixic acid combined with Methyl gallate and carvacrol may be microbiologically beneficial, rather than antagonists.
Environ Toxicol Pharmacol . 2014 Mar;37(2):626-37.
First report on isolation of methyl gallate with antioxidant, anti-HIV-1 and HIV-1 enzyme inhibitory activities from a mushroom (Pholiota adiposa)[Pubmed:
24572641]
Abstract
In this study, a compound with antioxidant and anti-HIV activities designated as HEB was first isolated from the edible mushroom Pholiota adiposa by extraction with ethanol and ethyl acetate. HEB was then purified by high performance liquid chromatography (HPLC) and identified to be Methyl gallate (C8H8O5, 184.1 Da) based on data from its mass spectrum (MS) and nuclear magnetic resonance (NMR) spectrum. HEB displayed strong antioxidant potency in inhibiting, at 1.36 mM concentration, erythrocyte hemolysis and scavenging DPPH radicals and superoxide anion (O2(-)) by 82.4%, 85.6% and 71.4%, respectively. Besides exhibiting a low cytotoxicity, compound HEB demonstrated significant anti-HIV activity in that it inhibited HIV-1 replication in TZM-BL cells infected by pseudovirus with an IC50 value of 11.9 μM. Further study disclosed that HEB inhibited the viral entry process and activities of key enzymes essential for the HIV-1 life cycle. HEB inhibited HIV-1 reverse transcriptase and integrase activities with an IC50 value of 80.1 μM and 228.5 μM, respectively, and at 10 mM concentration inhibited HIV-1 protease activity by 17.1% which was higher than that achieved by the positive control pepstatin A. Interestingly, this study first revealed that H2O2 stimulation not only activated cell oxidative stress responses, but also accelerated HIV-1 long terminal repeat (LTR) promotion in TZM-BL cells, which was significantly reduced by HEB from 18.2% to about 2%. It implied a direct relationship between the antioxidant and anti-HIV activities of the natural active constituent HEB. Nuclear transcription factor kappa B (NF-κB) signal pathways plays an important role in oxidative stress responses. Meanwhile, there is κB target sequence in HIV promoter LTR which is significant for virus replication and gene expression. In this study, Western Blot assay showed that HEB could inhibit the activation of NF-κB signal pathway stimulated by H2O2 in mouse spleen cells through suppressing NF-κB (p65) translocation into nucleus and NF-kappa-B inhibitor (IκB) degradation in cytoplasm. In summary, the antioxidant HEB from P. adiposa could inhibit HIV-1 replication through multiple target sites. The data suggest that natural antioxidant compounds might have a potential for treatment of AIDS.
Keywords: Antioxidant; HIV-1; Methyl gallate; Pholiota adipose.
J Immunol. 2010 Dec 1;185(11):6698-705.
Methyl gallate exhibits potent antitumor activities by inhibiting tumor infiltration of CD4+CD25+ regulatory T cells.[Pubmed:
21048105]
CD4(+)CD25(+) regulatory T (Treg) cells play crucial roles in the host response to tumors. Increasing evidence supports the existence of elevated numbers of Treg cells in solid tumors and hematologic malignancies.
METHODS AND RESULTS:
In this study, the effects of Methyl gallate on Treg cells were examined. Methyl gallate inhibited Treg cell-suppressive effects on effector CD4(+) T cells and Treg migration toward tumor environment. The expression of Treg surface markers including CTLA-4, CCR4, CXCR4, and glucocorticoid-induced TNFR was significantly suppressed upon Methyl gallate treatment. Furthermore, forkhead box P3 (Foxp3) expression was also significantly decreased by Methyl gallate, suggesting that the suppressive effects of Methyl gallate on Treg were medicated by decrease of Treg-specific transcription factor Foxp3. In tumor-bearing hosts, Methyl gallate treatment substantially reduced tumor growth and prolonged the survival rate. In contrast, nu/nu mice did not show decreased tumor progression in response to Methyl gallate. In addition, in tumor-bearing Treg-depleted mice, tumor growth and the survival rates were not changed by Methyl gallate treatment, strongly suggesting that the main therapeutic target of Methyl gallate in tumor suppression was related to modulation of the CD4(+)CD25(+) Treg cell functions. In the spleen of tumor-bearing mice, Methyl gallate treatment induced a significant decrease in the CD4(+)CD25(+)Foxp3(high) Treg cell population. Especially, the number of tumor-infiltrating CD25(+)Foxp3(high) Treg cells was significantly lower in Methyl gallate-treated mice.
CONCLUSIONS:
These results suggest that Methyl gallate can be used to reverse immune suppression and as a potentially useful adjunct for enhancing the efficacy of immune-based cancer therapy.
Arch Pharm Res. 2006 Oct;29(10):874-8.
Effects of methyl gallate on arachidonic acid metabolizing enzymes: Cyclooxygenase-2 and 5-lipoxygenase in mouse bone marrow-derived mast cells.[Pubmed:
17121182]
METHODS AND RESULTS:
Methyl gallate (MG) is a medicinal herbal product that is isolated from Paeonia lactiflora that inhibits cyclooxygenase-2 (COX-2) dependent phases of prostaglandin D2 (PGD2) generation in bone marrow-derived mast cells (BMMC) in a concentration-dependent manner with an IC50 values of 17.0 microM. This compound also found inhibited the COX-2-dependent conversion of the exogenous arachidonic acid to PGD2 in a dose-dependent manner with an IC50 values of 19.0 microM, using a COX enzyme assay kit. However, at concentrations up to 80 microM, MG did not inhibit COX-2 protein expression in BMMC, indicating that MG inhibits COX-2 activity directly. Furthermore, MG consistently inhibited the production of leukotriene C4 (LTC4) in a dose dependent manner, with an IC50 value of 5.3 microM.
CONCLUSIONS:
These results demonstrate that MG has a dual cyclooxygenase-2/5-lipoxygenase inhibitory activity, which might provide the basis for novel anti-inflammatory drugs.
Food Chem Toxicol. 2004 May;42(5):843-50.
Protective effect of methyl gallate from Toona sinensis (Meliaceae) against hydrogen peroxide-induced oxidative stress and DNA damage in MDCK cells.[Pubmed:
15046831]
Methyl gallate (MG) has been shown to be an effective antioxidant in a variety of acellular experiments.
METHODS AND RESULTS:
Accordingly, this study was designed to assess the ability of MG, extracting from Toona sinensis to protect cultured Madin-Darby canine kidney (MDCK) cells against hydrogen peroxide (H2O2)-mediated oxidative stress. Trolox, a cell permeable and water-soluble vitamin E analogue, was included for comparison. First, when MDCK cells were pretreated with MG and trolox for 1 h, followed by exposing to H2O2 (0.8 mM) for an additional hour, we found that the intracellular peroxide productions, as reflected by dichlorofluorescein (DCF) fluorescence, were shown to be decreased in a concentration-dependent manner. Furthermore, using C11-BODIPY581/591 as a lipid peroxidation probe, we also found that MG, in a concentration of 100 microM, could alleviate lipid peroxidation of the cells exposed to a short-term H2O2 treatment. In addition, MG-treated cells could prevent intracellular glutathione (GSH) from being depleted following an exposure of H2O2 (8.0 mM) for a 3 h period. Next, we also examined the effect of MG on H2O2-mediated oxidative damage to DNA. Using 8-oxoguanine as an indicator for oxidative DNA damage, we demonstrated that the percentage of MDCK cells containing 8-oxoguanine was drastically increased by exposing to H2O2 (40 mM) for 3 h. However, 8-oxoguanine contents were shown to be significantly decreased in the presence of MG prior to H2O2 exposure. Comparatively, MG was shown to be a better protective agent against oxidative damage to DNA as compared to trolox.
CONCLUSIONS:
Taken together, our data suggest that MG is effective in preventing H2O2-induced oxidative stress and DNA damage in MDCK cells. The underlying mechanisms involved scavenging of intracellular reactive oxygen species (ROS), inhibition of lipid peroxidation and prevention of intracellular GSH depletion.
Biosci Rep. 1988 Feb;8(1):95-102.
Methyl gallate, methyl-3,4,5-trihydoxybenzoate, is a potent and highly specific inhibitor of herpes simplex virus in vitro. II. Antiviral activity of methyl gallate and its derivatives.[Pubmed:
2840133]
METHODS AND RESULTS:
Methyl gallate (MG), methyl-3,4,5-trihydroxybenzoate, was highly active against herpes viruses as determined by plaque reduction assay. Herpes simplex virus type 2, MS strain, was sensitive to MG at a mean 50% inhibitory concentration (IC50) of 0.224 micrograms/ml in monkey kidney cells. MG was specific for herpes viruses with the relative sensitivity HSV-2 greater than HSV-1 greater than CMV. Two RNA viruses tested were significantly less sensitive to MG. The structural components of MG which modulate the anti-herpetic activity were identified by analysis of chemical analogues.
CONCLUSIONS:
Our structural analyses indicated that three hydroxyl groups were required but were not sufficient for the anti-herpetic action of MG. The presence and chain length of the alkyl ester were also important to the anti-herpetic activity of MG. Methyl gallate may interact with virus proteins and alter the adsorption and penetration of the virion.