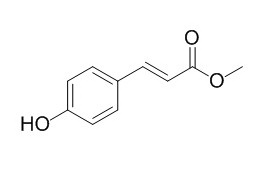
Methyl 4-hydroxycinnamate
Methyl 4-hydroxycinnamate is a model chromophore of the Photoactive Yellow Protein (PYP). Methyl 4-hydroxycinnamate can reduce IL-8 secretion at 10 ug/mL treatment concentrations; it may significantly contribute to antibacterial activities of Noni leaves.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Environ Toxicol.2021, 36(9):1848-1856.
J Mol Recognit.2020, 33(2):e2819
Molecules2022, 27(9):2827.
J Med Food.2021, 24(3):209-217.
Kor. J. Herbol.2022, 37(5): 89-96.
Chengdu University of Traditional Chinese Medicine2024, 4802935.
United States Patent Application2020, 20200038363
Front Immunol.2017, 8:1542
Eur J Neurosci.2021, 53(11):3548-3560.
J Cell Biochem.2024, 125(4):e30537.
Related and Featured Products
J Food Sci. 2016 May;81(5):M1192-6.
Antibacterial Constituents of Hainan Morinda citrifolia (Noni) Leaves.[Pubmed:
27074391]
Noni (Morinda citrifolia L.) is an edible and medicinal plant distributed in Hainan, China.
METHODS AND RESULTS:
The antibacterial activities of the extracts of water (WE), petroleum ether (PEE), ethyl acetate (EAE), chloroform (CE), and n-butanol (BE) were assayed by the disk diffusion method. The results showed that the extracts from Noni leaves possessed antibacterial effects against Bacillus subtilis, Escherichia coli, Proteus vulgaris, and Staphylococcus aureus. Among 5 different extracts, the BE produced the best antibacterial activity. The samples were first extracted by ethanol, and the primary compounds in the BE fraction of ethanol extract was further isolated and identified. Six phenolic compounds, including 5, 15-dimethylmorindol, ferulic acid, p-hydroxycinamic acid, methyl 4-hydroxybenzoate, methyl ferulate, and Methyl 4-hydroxycinnamate, were identifiedby NMR.
CONCLUSIONS:
The results indicated that the phenolic compounds might significantly contribute to antibacterial activities of Noni leaves.
J Food Sci. 2017 Aug;82(8):1792-1798.
Suppression of IL-8 Release by Sweet Olive Ethanolic Extract and Compounds in WiDr Colon Adenocarcinoma Cells.[Pubmed:
28671329]
Oxidative stress can stimulate the secretion of pro-inflammatory cytokines. Interleukin-8 (IL-8) has been implicated in the pathogenesis of inflammatory bowel disease and the metastatic spread of colorectal cancer. The flowers of Osmanthus fragrans (sweet olive) are used to alleviate dysentery with blood in the bowel, as well as stomach ache and diarrhea. However, the evidence of their therapeutic effects on these symptoms remains unclear.
METHODS AND RESULTS:
In the present study, the protective effects of sweet olive flower ethanolic extract (OFE) against oxidative stress in WiDr cells was assessed by evaluating its 1,1-diphenyl-2-picrylhydrazyl (DPPH) free radical scavenging activity. In addition, cellular IL-8 secretion was evaluated. Notably, high-performance liquid chromatography showed verbascoside to be the primary constituent in OFE; it exhibited a DPPH scavenging activity with an IC50 of 8.23 μg/mL. Moreover, OFE (1 to 100 μg/mL) showed a potent, dose-dependent inhibitory effect on H2 O2 -induced IL-8 secretion in WiDr cells. Nine compounds were isolated from OFE based on a protective effect-guided purification process. Of these compounds, 5 phenolic compounds-verbascoside, phillygenin, tyrosol, Methyl 4-hydroxycinnamate, and eutigoside A-reduced IL-8 secretion at 10 μg/mL treatment concentrations. Further analysis showed that the anti-inflammatory effects of OFE likely occurred via nuclear factor-κB pathway inhibition, which attenuates IL-8 secretion in cells.
CONCLUSIONS:
Collectively, these data suggest that OFE could be developed as an agent that suppresses IL-8 secretion to treat chronic inflammatory diseases.
J Phys Chem B. 2013 May 2;117(17):4798-805.
Conformational heterogeneity of methyl 4-hydroxycinnamate: a gas-phase UV-IR spectroscopic study.[Pubmed:
23574393]
UV excitation and IR absorption spectroscopy on jet-cooled molecules is used to study the conformational heterogeneity of Methyl 4-hydroxycinnamate, a model chromophore of the Photoactive Yellow Protein (PYP), and to determine the spectroscopic properties of the various conformers. UV-UV depletion spectroscopy identifies four different species with distinct electronic excitation spectra. Quantum chemical calculations argue that these species are associated with different conformers involving the s-cis/s-trans configuration of the ester with respect to the propenyl C-C single bond and the syn/anti orientation of the phenolic OH group. IR-UV hole-burning spectroscopy is used to record their IR absorption spectra in the fingerprint region. Comparison with IR absorption spectra predicted by quantum chemical calculations provides vibrational markers for each of the conformers, on the basis of which each of the species observed with UV-UV depletion spectroscopy is assigned. Although both DFT and wave function methods reproduce experimental frequencies, we find that calculations at the MP2 level are necessary to obtain agreement with experimentally observed intensities. To elucidate the role of the environment, we compare the IR spectra of the isolated conformers with IR spectra of Methyl 4-hydroxycinnamate-water clusters, and with IR spectra of Methyl 4-hydroxycinnamate in solution.
Phys Chem Chem Phys. 2012 Jul 7;14(25):8999-9005.
Nonradiative decay dynamics of methyl-4-hydroxycinnamate and its hydrated complex revealed by picosecond pump-probe spectroscopy.[Pubmed:
22684331]
The lifetimes of Methyl 4-hydroxycinnamate (OMpCA) and its mono-hydrated complex (OMpCA-H(2)O) in the S(1) state have been measured by picosecond pump-probe spectroscopy in a supersonic beam.
METHODS AND RESULTS:
For OMpCA, the lifetime of the S(1)-S(0) origin is 8-9 ps. On the other hand, the lifetime of the OMpCA-H(2)O complex at the origin is 930 ps, which is ∼100 times longer than that of OMpCA. Furthermore, in the complex the S(1) lifetime shows rapid decrease at an energy of ∼200 cm(-1) above the origin and finally becomes as short as 9 ps at ∼500 cm(-1). Theoretical calculations with a symmetry-adapted cluster-configuration interaction (SAC-CI) method suggest that the observed lifetime behavior of the two species is described by nonradiative decay dynamics involving trans → cis isomerization. That is both OMpCA and OMpCA-H(2)O in the S(1) state decay due to the trans → cis isomerization, and the large difference of the lifetimes between them is due to the difference of the isomerization potential energy curve.
CONCLUSIONS:
In OMpCA, the trans → cis isomerization occurs smoothly without a barrier on the S(1) surface, while in the OMpCA-H(2)O complex, there exists a barrier along the isomerization coordinate. The calculated barrier height of OMpCA-H(2)O is in good agreement with that observed experimentally.