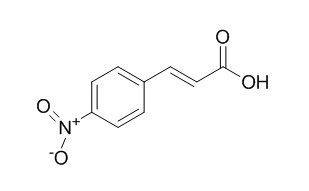
4-Nitrocinnamic acid is a photosensitive compound. 4-Nitrocinnamic acid has inhibitory effects on tyrosinase.
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
US 8758864 B2[P]. 2014.
Photosensitive semiconductor nanocrystals, photosensitive composition comprising semiconductor nanocrystals and method for forming semiconductor nanocrystal pattern using the same[Reference:
WebLink]
4. The organic-inorganic hybrid electroluminescent device according to claim 1, wherein the compound containing a photosensitive functional group is selected from a group consisting of methacrylic acid, crotonic acid, vinylacetic acid, tiglic acid, 3,3-dimethylacrylic acid, trans-2-pentenoic acid, 4-pentenoic acid, trans-2-methyl-2-pentenoic acid, 2,2-dimethyl-4-pentenoic acid, trans-2-hexenoic acid, trans-3-hexenoic acid, 2-ethyl-2-hexenoic acid, 6-heptenoic acid, 2-octenoic acid, citronellic acid, undecylenic acid, myristoleic acid, palmitoleic acid, oleic acid, elaidic acid, cis-11-elcosenoic acid, euric acid, nervonic acid, trans-2,4-pentadienoic acid, 2,4-hexadienoic acid, 2,6-heptadienoic acid, geranic acid, linoleic acid, 11,14-eicosadienoic acid, cis-8,11,14-eicosatrienoic acid, arachidonic acid, cis-5,8,11,14,17-eicosapentaenoic acid, cis-4,7,10,13,16,19-docosahexaenoic acid, fumaric acid, maleic acid, itaconic acid, ciraconic acid, mesaconic acid, trans-glutaconic acid, trans-beta-hydromuconic acid, trans-traumatic acid, trans-muconic acid, cis-aconitic acid, trans-aconitic acid, cis-3-chloroacrylic acid, trans-3-chloroacrylic acid, 2-bromoacrylic acid, 2-(trifluoromethyl)acryl-ic acid, trans-styrylacetic acid, trans-cinnamic acid, alpha.-methylcinnamic acid, 2-methylcinnamic acid, 2-fluorocinnamic acid, 2-(trifluoromethyl)cinnamic acid, 2-chlorocinnamic acid, 2-methoxycinnamic acid, 2-hydroxycinnamic acid, 2-nitrocinnamic acid, 2-carboxycinnamic acid, trans-3-fluorocinnamic acid, 3-(trifluoromethyl)cinnamic acid, 3-chlorocinnamic acid, 3-bromocinnamic acid, 3-methoxycinnamic acid, 3-hydroxycinnamic acid, 3-nitrocinnamic acid, 4-Methylcinnamic acid, 4-fluorocinnamic acid, trans-4-(trifluoromethyl)-cinnamic acid, 4-chlorocinnamic acid, 4-bromocinnamic acid, 4-methoxycinnamic acid, 4-hydroxycinnamic acid, 4-Nitrocinnamic acid, 3,3-dimethoxycinnamic acid, 4-vinylbenzoic acid, allyl methyl sulfide, allyl disulfide, diallyl amine, oleylamine, 3-amino-1-propanol vinyl ether, 4-chlorocinnamonitrile, 4-methoxycinnamonitrile, 3,4-dimethoxycinnamonitrile, 4-dimethylaminocinnamonitrile, acrylonitrile, allyl cyanide, crotononitrile, methacrylonitrile, cis-2-pentenenitrile, trans-3-pentenenitrile, 3,7-dimethyl-2,6-octadienenitrile, and 1,4-dicyano-2-butene.
J Biol Chem. 1985 Oct 5;260(22):11962-9.
Chloroperoxidase-catalyzed halogenation of trans-cinnamic acid and its derivatives.[Pubmed:
4044583 ]
Several 2,3-unsaturated carboxylic acids, such as trans-cinnamic acid and its derivatives, were found to be halogenated by chloroperoxidase of Caldariomyces fumago in the presence of hydrogen peroxide and either Cl- or Br-.
METHODS AND RESULTS:
Cinnamic acid, 4-hydroxycinnamic acid, 4-methoxycinnamic acid, and 3,4-dimethoxycinnamic acid were suitable substrates of chloroperoxidase, and were converted to 2-halo-3-hydroxycarboxylic acid, 2,3-dihydroxycarboxylic acid, decarboxylated halohydrin, or decarboxylated halocompound. However, 4-Nitrocinnamic acid and 4-chlorocinnamic acid having electron-attracting groups did not serve as a substrate of the enzyme. The enzyme also did not act on acrylic acid, acrylamide, crotonic acid, fumaric acid, etc.
CONCLUSIONS:
From these data, the enzymatic reactions of chloroperoxidase, concerning the substrate specificity, stereoselectivity, and the reaction mechanism, are discussed on the basis of current knowledge regarding the reaction mechanism of the enzyme. Also they are compared with the chemical reactions of molecular halogen and hypohalous acid.