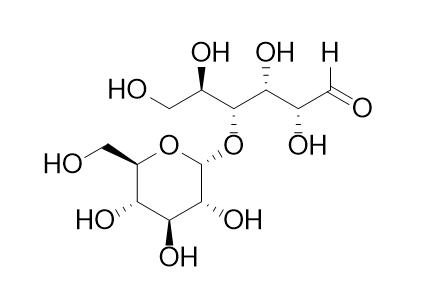
Maltose
Maltose is a dextrodisaccharide from malt and starch. It is used as a sweetening agent and fermentable intermediate in brewing.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Mol Cells.2018, 41(8):771-780
Sci Rep. 2017, 12953(7)
Appl. Sci.2020, 10(8),2804
Molecules2021, 26(1),230
Tissue Cell.2022, 78:101901.
BioResources J.2020, 15(3).
Phytother Res.2015, 29(7):1088-96
Pharmaceuticals (Basel).2021, 14(7):633.
Plant Cell Physiol.2018, 59(1):128-141
Molecules2022, 27(12):3824.
Related and Featured Products
Chem Asian J . 2021 Jul 19;16(14):1937-1941.
Enzymatic Hydrolysis-Responsive Supramolecular Hydrogels Composed of Maltose-Coupled Amphiphilic Ureas[Pubmed:
34003592]
Maltose is a ubiquitous disaccharide produced by the hydrolysis of starch. Amphiphilic ureas bearing hydrophilic Maltose moiety were synthesized via the following three steps: I) construction of urea derivatives by the condensation of 4-nitrophenyl isocyanate and alkylamines, II) reduction of the nitro group by hydrogenation, and III) an aminoglycosylation reaction of the amino group and the unprotected Maltose. These amphiphilic ureas functioned as low molecular weight hydrogelators, and the mixtures of the amphipathic ureas and water formed supramolecular hydrogels. The gelation ability largely depended on the chain length of the alkyl group of the amphiphilic urea; amphipathic urea having a decyl group had the highest gelation ability (minimum gelation concentration=0.4 mM). The physical properties of the supramolecular hydrogels were evaluated by measuring their thermal stability and dynamic viscoelasticity. These supramolecular hydrogels underwent gel-to-sol phase transition upon the addition of α-glucosidase as a result of the α-glucosidase-catalyzed hydrolysis of the Maltose moiety of the amphipathic urea.
Biosci Biotechnol Biochem . 2004 Jan;68(1):91-95.
Decomposition kinetics of maltose in subcritical water[Pubmed:
14745169]
The decomposition process of Maltose in subcritical water was studied using a tubular reactor in the temperature range of 180 to 260 degrees C and at 10 MPa. The formation of glucose and 5-hydroxymethyl-2-furaldehyde during the Maltose decomposition was also observed. The decomposition rate of Maltose was faster at higher temperatures. The rate was approximated by first-order kinetics during the early stage of the decomposition, but was accelerated and deviated from these kinetics at the later stage. The effluent pH decreased as the residence time in the reactor increased and the decrease of pH affected the Maltose decomposition rate and glucose formation. Low pH of a feed solution accelerated Maltose decomposition. A good correlation was obtained between the pH of the effluent and the rate constant of the first-order kinetics.
Am J Clin Nutr . 1990 Oct;52(4):689-693.
Metabolism of intravenously administered maltose in renal tubules in humans[Pubmed:
2403061]
To investigate how urinary excretion rates (UERs) of Maltose and glucose are determined after intravenous Maltose infusion, Maltose and glucose solutions were infused at various rates and the relationships between UERs of Maltose and glucose and their plasma concentrations were examined. Results showed the existence of a threshold plasma Maltose concentration for the urinary excretions of Maltose and glucose and the existence of a maximum rate of urinary glucose excretion after Maltose infusion. Elevation of plasma glucose concentration by simultaneous glucose infusion increased urinary glucose excretion but did not increase urinary Maltose excretion; the relationship between plasma total sugar concentration and urinary total sugar excretion was unchanged. Results suggest that Maltose administered intravenously is hydrolyzed to glucose by maltase in renal tubules and reabsorbed as glucose competitively with glucose derived from plasma and that the maximum utilization of intravenously infused Maltose is determined by the tubular glucose reabsorption capacity.
Z Ernahrungswiss . 1976 Sep;15(3):270-283.
[Animal experiment studies on parenteral utilization of maltose][Pubmed:
135420]
The utilisation of parenterally administered Maltose was investigated in the anaesthetized rat, and in rats fixed in metabolic cages. Additionally, the metabolism of Maltose was measured with the isolated perfused rat liver. During intravenous infusion of 0.3 g, 0.6 g or 1.2 g Maltose (corresponding to 0.9 g, 1.8 g or 3.6 g/kg bodyweight) per hour a steady-state for Maltose in blood was attained. Blood glucose concentration rose during the Maltose infusions. The utilisation of parenterally administered Maltose was established by a high rate of glycogen storage in the liver and by a decrease in concentration of free fatty acids in serum. During the 72 hour infusion of Maltose at a rate of 0.23 g/hour (corresponding to 0.70 g/kg bodyweight) a constant blood Maltose concentration of 60 mg/100 ml was measured. Simultaneously, the blood glucose concentration increased. The excretion of Maltose and of glucose was approximately 5% of the total amount administered intravenously. The nitrogen sparing effect of Maltose was equal to that of glucose (or glucose substitutes). In the streptozotocindiabetic rats, the renal excretion of Maltose and glucose was 20-30% of the total amount. Moreover, blood glucose concentration was elevated significantly during Maltose infusion. In the isolated perfused rat liver Maltose hydrolysis was established. However, the glucose obtained by this hydrolysis was not metabolized by the isolated organ as was observed for glucose. On the other hand, the glucose substitutes (fructose, xylitol, sorbitol) are also partially transformed to glucose by the isolated liver. These substances, however, were additionally utilized by this organ in other ways. On the basis of these results it is concluded that Maltose is metabolized following its intravenous application. Therefore, Maltose should have advantages compared to glucose because of the lower osmotic pressure. The metabolism of Maltose, however, is similar to that of glucose. The advantages of the glucose substitutes (fructose, sorbitol, xylitol) are not shared by Maltose.