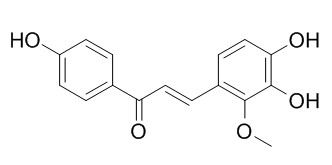
Licochalcone B
Licochalcone B has antitumor, antimetastatic, cardioprotective, antioxidant, antiapoptotic, and anti-inflammatory effects, it can significantly inhibit LPS-induced phosphorylation at serine 276 and transcriptional activation of NF-KB. Licochalcone B can protect the liver from carbon tetrachloride (CCl4)-induced injury, the protection may be due to inhibition of p38 and NFκB signaling, which subsequently reduces inflammation in the liver.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
J Cachexia Sarcopenia Muscle.2022, 13(6):3149-3162.
Applied Biological Chemistry 2021, 64(75)
Fitoterapia.2024, 175:105955.
J Biomol Struct Dyn.2024, 1-12.
Separations2023, 10(7), 411.
J. Korean Wood Sci. Technol.2022, 50(5):338-352.
Sichuan Agricultural University2023, 4630743.
Scientific World Journal.2014, 2014:654193
Journal of Natural Remedies2024, 24(3):555�C575.
J Ethnopharmacol.2023, 309:116302.
Related and Featured Products
Okanin
Catalog No: CFN90849
CAS No: 484-76-4
Price: $318/20mg
Sappanchalcone
Catalog No: CFN97522
CAS No: 94344-54-4
Price: $318/10mg
Robtein
Catalog No: CFN97767
CAS No: 2679-65-4
Price: Inquiry(manager@chemfaces.com)
6'-Hydroxy-3,4,5,2',3',4'-Hexamethoxychalcone
Catalog No: CFN91493
CAS No: 1818307-98-0
Price: Inquiry(manager@chemfaces.com)
Naringenin chalcone
Catalog No: CFN90606
CAS No: 73692-50-9
Price: $168/20mg
Homoeriodictyol chalcone
Catalog No: CFN91406
CAS No: 52218-19-6
Price: Inquiry(manager@chemfaces.com)
2'-Hydroxy-3,4,4',6'-tetramethoxychalcone
Catalog No: CFN91403
CAS No: 10496-67-0
Price: Inquiry(manager@chemfaces.com)
2-Hydroxy-3,4,5,6-tetramethoxychalcone
Catalog No: CFN97758
CAS No: 219298-74-5
Price: Inquiry(manager@chemfaces.com)
Echinatin
Catalog No: CFN99520
CAS No: 34221-41-5
Price: $198/20mg
Loureirin C
Catalog No: CFN92858
CAS No: 116384-24-8
Price: $268/10mg
Food Chem Toxicol. 2014 Mar;65:242-51.
Licochalcone B inhibits growth of bladder cancer cells by arresting cell cycle progression and inducing apoptosis.[Pubmed:
24384411]
To examine the mechanisms by which Licochalcone B (LCB) inhibits the proliferation of human malignant bladder cancer cell lines (T24 and EJ) in vitro and antitumor activity in vivo in MB49 (murine bladder cancer cell line) tumor model.
METHODS AND RESULTS:
Exposure of T24 or EJ cells to LCB significantly inhibited cell lines proliferation in a concentration-dependent and time-dependent manner, and resulted in S phase arrest in T24 or EJ cells, respectively. LCB treatment decreased the expression of cyclin A, cyclin-dependent kinase (CDK1 and CDK2) mRNA, cell division cycle 25 (Cdc25A and Cdc25B) protein. In addition, LCB treatment down-regulated Bcl-2 and survivin expression, enhanced Bax expression, activated caspase-3 and cleaved poly (ADP-ribose) polymerase (PARP) protein. Consistently, the tumorigenicity of LCB-treated MB49 cells was limited significantly by using the colony formation assay in vitro and the MB49 tumor model performed in C57BL/6 mice in vivo.
CONCLUSIONS:
These findings provide support for the use of LCB in chemoprevention and bladder cancer therapy.
Int Immunopharmacol. 2009 Apr;9(4):499-507.
Glycyrrhiza inflata-derived chalcones, Licochalcone A, Licochalcone B and Licochalcone D, inhibit phosphorylation of NF-kappaB p65 in LPS signaling pathway.[Pubmed:
19291859]
Licorice root has been used as a traditional medicine for the treatment of gastric ulcer, bronchial asthma and inflammation. Licochalcone A is a major component of Xinjiang licorice, Glycyrrhiza inflata.
METHODS AND RESULTS:
Previously we showed that Licochalcone A significantly inhibited LPS-induced NF-kappaB transcriptional activation by abrogating the phosphorylation of NF-kappaB p65 at serine 276. Glycyrrhiza inflata contains not only Licochalcone A but also Licochalcone B, Licochalcone C, Licochalcone D, Echinatin and Isoliquiritigenin, harboring the common structure of chalcones. No chalcones had any effect on LPS-induced IkappaB degradation, nuclear translocation and DNA binding activity of NF-kappaB p65; however, we observed that Licochalcone B and Licochalcone D significantly inhibited LPS-induced phosphorylation at serine 276 and transcriptional activation of NF-kappaB, the same as Licochalcone A. Interestingly, we also found that Licochalcone A, Licochalcone B and Licochalcone D effectively inhibited LPS-induced activation of PKA, which is required for the phosphorylation of NF-kappaB p65 at serine 276. Consequently, Licochalcone B and Licochalcone D significantly reduced the LPS-induced production of NO, TNFalpha and MCP-1. On the other hand, Licochalcone C, Echinatin and Isoliquitigenin failed to inhibit LPS-induced NF-kappaB activation.
CONCLUSIONS:
These findings suggest that the anti-inflammatory effect of Glycyrrhiza inflata is ascribable to the potent inhibition of NF-kappaB by Licochalcone A, Licochalcone B and Licochalcone D.
Nat Prod Res . 2020 Mar;34(5):736-739.
Licochalcone B, a chalcone derivative from Glycyrrhiza inflata, as a multifunctional agent for the treatment of Alzheimer's disease[Pubmed:
30345819]
Abstract
Licochalcone B (LCB), an extract from the root of Glycyrrhiza inflate, has the same caffeic acid scaffold as curcumin (Cur), which is known as an anti-Alzheimer's disease (AD) agent. However, there is no relevant research about anti-AD activity of LCB. In this study, the anti-AD activity of LCB was investigated. LCB could inhibit amyloid beta (Aβ42) self-aggregation (IC50 = 2.16 ± 0.24 μM) and disaggregate pre-formed Aβ42 fibrils, reduce metal-induced Aβ42 aggregation through chelating metal ions. Molecular docking further revealed that LCB inhibited Aβ42 self-aggregation through forming two hydrogen bonds with Lys28 to block the salt bridge interaction at the C-terminus of Aβ42. Anti-oxidant property of LCB was also observed by DCFH-DA assay. In addition, LCB did show neuroprotective activity against H2O2-induced cell death in SH-SY5Y cells. In general, our results demonstrate that LCB, as a multifunctional agent, is likely to be promising therapeutics for AD.
Phytomedicine . 2019 Oct;63:153014.
Licochalcone B inhibits growth and induces apoptosis of human non-small-cell lung cancer cells by dual targeting of EGFR and MET[Pubmed:
31323446]
Abstract
Background: Epidermal growth factor receptor (EGFR) gene alterations are associated with sensitization to tyrosine kinase inhibitors such as gefitinib in lung cancer. Some patients suffering from non-small cell lung cancer (NSCLC) have difficulty in treating the cancer due to resistance acquired to gefitinib with MET amplification. Therefore EGFR and MET may be attractive targets for lung cancer therapy.
Purpose: This study aimed to investigate the anti-cancer activity of Licochalcone (LC)B extracted from Glycyrrhiza inflata, in gefitinib-sensitive or gefitinib-resistant NSCLC cells, and to define its mechanisms.
Study design: We investigated the mechanism of action of LCB by targeting EGFR and MET in human NSCLC cells.
Methods: We used the HCC827 and HCC827GR lines as gefitinib-sensitive and -resistant cells respectively, and determined the effects of LCB on both, by performing cell proliferation assay, flow cytometry analysis and Western blotting. Targets of LCB were identified by pull-down/kinase assay and molecular docking simulation.
Results: LCB inhibited both EGFR and MET kinase activity by directly binding to their ATP-binding pockets. The ability of this interaction was verified by computational docking and molecular dynamics simulations. LCB suppressed viability and colony formation of both HCC827 and HCC827GR cells while exhibiting no cytotoxicity to normal cells. The induction of G2/M cell-cycle arrest and apoptosis by LCB was confirmed by Annexin V/7-AAD double staining, ER stress and reactive oxygen species induction, mitochondrial membrane potential loss and caspase activation as well as related-proteins regulation. Inhibition of EGFR and MET by LCB decreased ERBB3 and AKT axis activation.
Conclusion: We provide insights into the LCB-mediated mechanisms involved in reducing cell proliferation and inducing apoptosis in NSCLC cells. This occurs through dual inhibition of EGFR and MET in NSCLC cells regardless of their sensitivity or resistance to gefitinib. LCB may be a promising novel therapeutic medicine for gefitinib-sensitive or resistant NSCLC treatment.
Keywords: Apoptosis; EGFR; Licochalcone B; MET; Non-small cell lung cancer.
Chem Biol Interact. 2014 Oct 29;224C:142-148.
(E)-3-(3,4-dihydroxy-2-methoxyphenyl)-1-(2,4-dihydroxyphenyl)prop-2-en-1-one, a novel licochalcone B derivative compound, suppresses lipopolysaccharide-stimulated inflammatory reactions in RAW264.7 cells and endotoxin shock in mice.[Pubmed:
25451593]
Activated macrophages mediate inflammation, as they release nitric oxide and pro-inflammatory cytokines in various inflammatory diseases. Suppressing macrophage activation may alleviate inflammatory processes.
METHODS AND RESULTS:
Here, we report that (E)-3-(3,4-dihydroxy-2-methoxyphenyl)-1-(2,4-dihydroxyphenyl)prop-2-en-1-one (DDP), a novel Licochalcone B derivative compound, inhibits inflammatory reactions in macrophages and protects mice from endotoxin shock. In vitro experiments showed that DDP suppressed the generation of nitric oxide and pro-inflammatory cytokines by suppressing the activation of nuclear factor-κB and activator protein-1 and simultaneously inhibited its upstream inflammatory signaling cascades in lipopolysaccharide in RAW264.7 cells. In an animal model, DDP protected BALB/c mice from lipopolysaccharide-induced endotoxin shock, possibly through inhibition of the production of inflammatory cytokines.
CONCLUSIONS:
DDP inhibited the production of inflammatory mediators and may be a potential target for treatment of various inflammatory diseases.
Oxid Med Cell Longev. 2014;2014:134862.
Cardioprotection against ischemia/reperfusion by licochalcone B in isolated rat hearts.[Pubmed:
25215172]
The generation of reactive oxygen species (ROS) is a major cause of heart injury induced by ischemia-reperfusion. The left ventricular developed pressure (LVDP) and the maximum up/down rate of left ventricular pressure (±dp/dt(max)) were documented by a physiological recorder.
METHODS AND RESULTS:
Myocardial infarct size was estimated macroscopically using 2,3,5-triphenyltetrazolium chloride staining. Coronary effluent was analyzed for lactate dehydrogenase (LDH) and creatine kinase (CK) release to assess the degree of cardiac injury. The levels of C-reactive protein (CRP), interleukin-8 (IL-8), tumor necrosis factor-α (TNF-α), and interleukin-6 (IL-6) were analyzed to determine the inflammation status of the myocardial tissue. Cardiomyocyte apoptosis analysis was performed using the In Situ Cell Death Detection Kit, POD. Accordingly, Licochalcone B pretreatment improved the heart rate (HR), increased LVDP, and decreased CK and LDH levels in coronary flow. SOD level and GSH/GSSG ratio increased, whereas the levels of MDA, TNF-α, and CRP and activities of IL-8 and IL-6 decreased in Licochalcone B-treated groups. The infarct size and cell apoptosis in hearts from Licochalcone B-treated group were lower than those in hearts from the I/R control group.
CONCLUSIONS:
Therefore, the cardioprotective effects of Licochalcone B may be attributed to its antioxidant, antiapoptotic, and anti-inflammatory activities.
EMBO Rep . 2022 Feb 3;23(2):e53499.
Licochalcone B specifically inhibits the NLRP3 inflammasome by disrupting NEK7-NLRP3 interaction[Pubmed:
34882936]
Abstract
The activation of the nucleotide oligomerization domain (NOD)-like receptor (NLR) family, pyrin domain-containing protein 3 (NLRP3) inflammasome is related to the pathogenesis of a wide range of inflammatory diseases, but drugs targeting the NLRP3 inflammasome are still scarce. In the present study, we demonstrated that Licochalcone B (LicoB), a main component of the traditional medicinal herb licorice, is a specific inhibitor of the NLRP3 inflammasome. LicoB inhibits the activation of the NLRP3 inflammasome in macrophages but has no effect on the activation of AIM2 or NLRC4 inflammasome. Mechanistically, LicoB directly binds to NEK7 and inhibits the interaction between NLRP3 and NEK7, thus suppressing NLRP3 inflammasome activation. Furthermore, LicoB exhibits protective effects in mouse models of NLRP3 inflammasome-mediated diseases, including lipopolysaccharide (LPS)-induced septic shock, MSU-induced peritonitis and non-alcoholic steatohepatitis (NASH). Our findings indicate that LicoB is a specific NLRP3 inhibitor and a promising candidate for treating NLRP3 inflammasome-related diseases.
Keywords: LPS-induced septic shock; Licochalcone B; MSU-induced peritonitis; NASH; NEK7; NLRP3 inflammasome.
Basic Clin Pharmacol Toxicol. 2014 Dec;115(6):527-33.
Antimetastatic effects of licochalcone B on human bladder carcinoma T24 by inhibition of matrix metalloproteinases-9 and NF-кB activity.[Pubmed:
25099010]
This study investigated the mechanisms by which Licochalcone B (LCB) inhibits the adhesion,invasion and metastasis of human malignant bladder cancer T24 cells.
METHODS AND RESULTS:
Cell viability was evaluated using a sulforhodamine B (SRB) assay. Cell migration and invasion ability were conducted using wound-healing assay and matrigel transwell invasion assay. The activities of matrix metalloproteinases (MMP)-2 and MMP-9 were measured by gelatin zymography protease assays. The expression in protein level of NF-κBP65 and AP-1 was determined using the ELISA method; the protein levels of MMP-9, NF-κBP65, IκBα and P-IκBα were detected by Western blot. The expression in mRNA level of MMP-9 was assessed using quantitative real-time polymerase chain reaction (PCR) and reverse transcription PCR. The results indicated that Licochalcone Battenuated T24 cell migration, adhesion and invasion in a concentration-dependent manner. Licochalcone B treatment down-regulated the mRNA expression, protein expression and activity of MMP-9 but had no effect on MMP-2. In addition, Licochalcone B treatment decreased the protein level of NF-кBP65 and nuclear translocation of NF-кB. These findings suggested that Licochalcone Battenuated migration of bladder cancer T24 cells and adhesion and invasion accompanied with down-regulated protein expression of MMP-9 and the nuclear translocation of NF-кB.
CONCLUSIONS:
Our results provide support that LCB may be a potent adjuvant therapeutic agent in the prevention and therapy of bladder cancer.
Iran J Basic Med Sci. 2016 Aug;19(8):910-5.
Hepatoprotective effects of licochalcone B on carbon tetrachloride-induced liver toxicity in mice.[Pubmed:
27746874 ]
The objective of this study was to investigate the hepatoprotective effect of Licochalcone B (LCB) in a mice model of carbon tetrachloride (CCl4)-induced liver toxicity.
METHODS AND RESULTS:
CCl4-induced hepatotoxicity was manifested by an increase in the levels of ALT, AST, MDA, IL-6, CRP, and TNF-ɑ, and a decrease in the SOD level and GSH/GSSG ratio in the serum. The histopathological examination of the liver sections revealed necrosis and inflammatory reactions. Pretreatment with LCB decreased the levels of ALT, AST, MDA, GSSG, IL-6, CRP, TNF-ɑ, and the protein expression of p38 and NF-κB, increased the level of SOD and GSH, and normalized the hepatic histo-architecture.
CONCLUSIONS:
LCB protected the liver from CCl4-induced injury. Protection may be due to inhibition of p38 and NFκB signaling, which subsequently reduced inflammation in the liver.