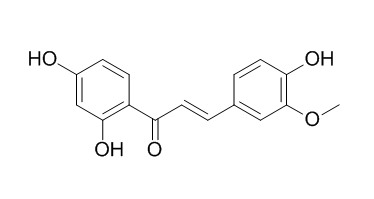
Homobutein
Homobutein as a dual inhibitors of HDACs and NF-κB, it can serve as a lead compound in the development of dual inhibitors against both targets in the treatment of inflammation and cancer. Homobutein shows antioxidant and antimalarial properties based on its ability to chelate iron (II and III) cations.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Asian Journal of Chemistry2018, 30(12):2699-2703
Molecules.2022, 27(7):2093.
Microorganisms.2021, 9(12):2514.
Antioxidants (Basel).2024, 13(8):951.
bioRxiv2021, 458409.
PLoS One.2020, 15(2):e0220084.
J Ethnopharmacol.2020, 249:112396
Molecules.2019, 24(22):E4022
Int J Mol Sci.2018, 19(9):E2681
Planta Med.2022, a-1876-3009.
Related and Featured Products
Oncology Reports, 15 Jun 2012, 28(3):797-805.
Natural chalcones as dual inhibitors of HDACs and NF-κB.[Reference:
WebLink]
Histone deacetylase enzymes (HDACs) are emerging as a promising biological target for cancer and inflammation.
METHODS AND RESULTS:
Using a fluorescence assay, we tested the in vitro HDAC inhibitory activity of twenty-one natural chalcones, a widespread group of natural products with well-known anti-inflammatory and antitumor effects. Since HDACs regulate the expression of the transcription factor NF-κB, we also evaluated the inhibitory potential of the compounds on NF-κB activation. Only four chalcones, isoliquiritigenin (no. 10), butein (no. 12), Homobutein (no. 15) and the glycoside marein (no. 21) showed HDAC inhibitory activity with IC50 values of 60-190 µM, whereas a number of compounds inhibited TNFα-induced NF-κB activation with IC50 values in the range of 8-41 µM. Interestingly, three chalcones (nos. 10, 12 and 15) inhibited both TNFα-induced NF-κB activity and total HDAC activity of classes I, II and IV. Molecular modeling and docking studies were performed to shed light into dual activity and to draw structure-activity relationships among chalcones (nos. 1-21). To the best of our knowledge this is the first study that provides evidence for HDACs as potential drug targets for natural chalcones. The dual inhibitory potential of the selected chalcones on NF-κB and HDACs was investigated for the first time.
CONCLUSIONS:
This study demonstrates that chalcones can serve as lead compounds in the development of dual inhibitors against both targets in the treatment of inflammation and cancer.
European Food Research & Technology, 2016, 242(1):71-90.
Antioxidant and antimalarial properties of butein and homobutein based on their ability to chelate iron (II and III) cations: a DFT study in vacuo and in solution[Reference:
WebLink]
A theoretical study on the antioxidant and antimalarial properties of butein and Homobutein has been performed by considering their Fe2+ and Fe3+ chelation ability. In order to elucidate the origin of the antioxidant and antimalarial properties of these compounds, the study attempts to investigate the nature of the complex structures, ligand···Fen+ stabilities and electronic properties of the Fe cations before and after complexation.
METHODS AND RESULTS:
The investigation considered the neutral and the deprotonated species of butein and Homobutein interacting with the Fe cations as well as the deprotonated species of butein and Homobutein interacting with micro-solvated Fe2+ or Fe3+ cation. The study has been performed using B3LYP/6-31+G(d,p) method. The LANL2DZ pseudo-potential was selected to describe the Fe cations. Final energies were obtained using the B3LYP/6-311+G(2d,p)//B3LYP/6-31+G(d,p) method. The binding energies depend on the media (it is higher in vacuo than in water solution), the nature of the cation (it is higher for Fe3+ than for Fe2+), the nature of the ligand and the Fen+ coordination site on the ligand (it is highest for the bidentate Fe coordination to O2′ and O9 atoms and lowest for the Fe coordination on the π system of the aromatic ring). The charge on Fen+ decreases on coordination to the ligand.
CONCLUSIONS:
AIM analysis suggests that the strong cation···ligand interactions are more likely covalent than ionic in vacuo and entirely ionic in solution. The ability of the ligands to reduce the Fe cation coupled with the strong iron-binding properties has significant implication on their antioxidant and antimalarial activities.