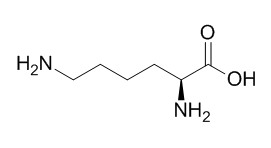
L-lysine
Lysine is an essential amino acid for the human body. L-lysine and L-glutamic acid and as useful building blocks for the preparation of bifunctional DTPA-like ligands.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Front Nutr.2023, 10:1181135.
Mol Pharmacol.2021, 99(2):163-174.
Biomedicines.2022, 10(2):463.
Evid Based Complement Alternat Med.2018, 2018:8565132
Chinese J of Tissue Engineering Res.2022, 26(17): 2636-2641.
RSC Adv.2023, 13(9):6317-6326.
J Control Release.2021, 336:159-168.
Food Bioscience2024, 58:103691.
LWT-Food Science and Technology2017, 75:488-496
Food Chem.2020, 332:127412
Related and Featured Products
Appl Microbiol Biotechnol. 1998 Jan;49(1):24-30.
Improved L-lysine yield with Corynebacterium glutamicum: use of dapA resulting in increased flux combined with growth limitation.[Pubmed:
9487706]
The amino acid L-lysine is produced on a large scale using mutants of Corynebacterium glutamicum. However, as yet recombinant DNA techniques have not succeed in improving strains selected for decades by classic mutagenesis for high productivity.
METHODS AND RESULTS:
We here report that seven biosynthetic enzymes were assayed and oversynthesis of the dihydrodipicolinate synthase resulted in an increase of lysine accumulation from 220 mM to 270 mM. The synthase, encoded by dapA, is located at the branch point of metabolite distribution to either lysine or threonine and competes with homoserine dehydrogenase for the common substrate aspartate semialdehyde. When graded dapA expression was used, as well as quantification of enzyme activities, intracellular metabolite concentrations and flux rates, a global response of the carbon metabolism to the synthase activity became apparent: the increased flux towards lysine was accompanied by a decreased flux towards threonine. This resulted in a decreased growth rate, but increased intracellular levels of pyruvate-derived valine and alanine.
CONCLUSIONS:
Therefore, modulating the flux at the branch point results in an intrinsically introduced growth limitation with increased intracellular precursor supply for lysine synthesis. This does not only achieve an increase in lysine yield but this example of an intracellularly introduced growth limitation is proposed as a new general means of increasing flux for industrial metabolite over-production.
BMC Complement Altern Med . 2015 Jun 23;15:193.
Suppression of acute pancreatitis by L-lysine in mice[Pubmed:
26100532]
Abstract
Background: Acute pancreatitis is an inflammatory disease caused by several factors such as viral infection, drugs, and diagnostic endoscopy. The aim of this study was to evaluate the potential protective or therapeutic effects of L-lysine on pancreatitis induced by L-arginine in mice.
Methods: Four groups of mice (10 in each group) were assessed. Group I was the control. Animals in groups II-IV were injected intraperitoneally with L-arginine hydrochloride (400 mg/kg body weight [bw]) for 3 days. Group III animals were orally pre-treated with L-lysine (10 mg/kg bw), whereas group IV animals were orally post-treated with L-lysine (10 mg/kg bw). Serum samples were subjected to amylase, lipase, transaminase, and interleukin-6 (IL-6) assays. The pancreas was excised to measure the levels of malondialdehyde, nitric oxide, catalase, superoxide dismutase, reduced glutathione, and glutathione peroxidase.
Results: Pre- or post-treatment with L-lysine led to significant decreases in the levels of malondialdehyde and nitric oxide, while significant enhancement was observed in the activities of antioxidant enzymes (superoxide dismutase, catalase, and glutathione peroxidase) and glutathione (p < 0.001). However, the treatment potential of L-lysine was better as a protective agent than a therapeutic agent.
Conclusions: L-lysine treatment attenuates pancreatic tissue injury induced by L-arginine by inhibiting the release of the inflammatory cytokine IL-6 and enhance antioxidant activity. These effects may involve upregulation of anti-inflammatory factors and subsequent downregulation of IL6.
Acta Cir Bras . 2017 Apr;32(4):297-306.
Transitional metaplasia in intestinal epithelium of rats submitted to intestinal cystoplasty and treatment with L -lysine[Pubmed:
28538804]
Abstract
Purpose:: To evaluated the effects of L-lysine on the intestinal and urothelial epithelia in cystoplasty in rats.
Methods:: Twenty-eight 9-week-old rats were assigned to 4 groups: Group A (n=8) cystoplasty followed by administration of L-lysine (150 mg/kg body weight by gavage) for 30 weeks; Group B (n=8) cystoplasty + water for 30 weeks; Group C (n=6) L-lysine for 30 weeks; Group D (n=6) water for 30 weeks.
Results:: On histopathology with hematoxylin and eosin, mild to moderate hyperplasia transitional was observed in at the site of anastomosis in all animals submitted to cystoplasty (Groups A and B), but "transitional metaplasia" of the intestinal glandular epithelium was more accentuated in Group A (p=0.045). No inflammatory cells, dysplasia or abnormalities were observed. Staining with Alcian blue revealed a substantial reduction of goblet cells and mucins in the colon segment (Groups A and B).
Conclusion:: The administration of L-lysine to rats accelerated the development of transitional metaplasia in the epithelium of the colon segment in cystoplasty.
J Ind Microbiol Biotechnol. 2006 Jul;33(7):610-5.
A genome-based approach to create a minimally mutated Corynebacterium glutamicum strain for efficient L-lysine production[Pubmed:
16506038 ]
METHODS AND RESULTS:
Based on the progress in genomics, we have developed a novel approach that employs genomic information to generate an efficient amino acid producer. A comparative genomic analysis of an industrial L-lysine producer with its natural ancestor identified a variety of mutations in genes associated with L-lysine biosynthesis. Among these mutations, we identified two mutations in the relevant terminal pathways as key mutations for L-lysine production, and three mutations in central metabolism that resulted in increased titers. These five mutations when assembled in the wild-type genome led to a significant increase in both the rate of production and final L-lysine titer. Further investigations incorporated with transcriptome analysis suggested that other as yet unidentified mutations are necessary to support the L-lysine titers observed by the original production strain.
CONCLUSIONS:
Here we describe the essence of our approach for strain reconstruction, and also discuss mechanisms of L-lysine hyperproduction unraveled by combining genomics with classical strain improvement.
Bioconjug Chem. 1999 Jan-Feb;10(1):137-40.
L-Glutamic acid and L-lysine as useful building blocks for the preparation of bifunctional DTPA-like ligands.[Pubmed:
9893975 ]
METHODS AND RESULTS:
Bisalkylation of suitably protected L-glutamic acid and L-lysine derivatives with tert-butyl N-(2-bromoethyl)iminodiacetate 2, followed by deprotection of the omega functional group affords N, N-bis[2-[bis[2-(1, 1-dimethylethoxy)-2-oxoethyl]amino]ethyl]-L-glutamic acid 1-(1, 1-dimethylethyl) ester 4 and N2,N2-bis[2-[bis[2-(1, 1-dimethylethoxy)-2-oxoethyl]amino]ethyl]-L-lysine 1,1-dimethylethyl ester 7. Such compounds feature a carboxylic or an amino group, respectively, which are available for conjugation with a suitable partner via formation of an amide bond.
CONCLUSIONS:
The conjugates, which can be prepared in this way, contain a chelating subunit in which all five acetic residues of DTPA are available for the complexation of metal ions. Direct bisalkylation of glycine with 2 promptly gives N, N-bis[2-[bis[2-(1,1-dimethylethoxy)-2-oxoethyl]amino]ethyl]glycine 11. The latter allows to achieve conjugates in which the central acetic group of DTPA is selectively converted into an acetamide.