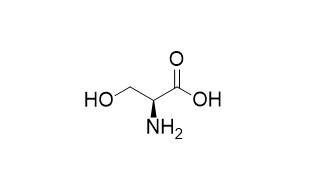
L-Serine
L-Serine and glycine mediate the trophic actions of glial cells on Purkinje neurons. L-serine, and D-serine levels have been studied, since they could serve as biological markers.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Curr Issues Mol Biol.2024, 46(4):3328-3341.
Prev Nutr Food Sci.2025, 30(1):92-100.
Antioxidants (Basel).2020, 9(6):544.
Front Pharmacol.2022, 13:972825.
Arch Biochem Biophys.2020, 687:108384.
Pharm Biol.2021, 59(1):134-145.
Molecular & Cellular Toxicology 2024, 00444-8.
Phytomedicine.2018, 47:48-57
Korean J. Food Sci. & Technol.2022, 54(2):241-246
Oxid Med Cell Longev.2022, 2022:5888636.
Related and Featured Products
Nature, 1988, 331(6158):723-725.
Gene for a Novel tRNA Species That Accepts L-serine and Cotranslationally Inserts Selenocysteine[Reference:
WebLink]
The biological requirement of the trace element selenium was recognized 40 years ago. Selenium is incorporated into several enzymes and transfer RNA species of both prokaryotic and eukaryotic origin. In enzymes which contain a selenopolypeptide, selenium is present as covalently bound selenocysteine which participates in the catalytic reaction. Sequence analysis of the genes coding for two selenoproteins, formate dehydrogenase H from Escherichia coli and glutathione peroxidase from mouse and man, demonstrated that an in-frame UGA opal nonsense codon directs the incorporation of selenocysteine. In the case of formate dehydrogenase incorporation occurs cotranslationally.
METHODS AND RESULTS:
Recently, we identified four genes whose products are required for selenocysteine incorporation in E. coli. We report here that one of these genes codes for a tRNA species with unique properties. It possesses an anticodon complementary to UGA and deviates in several positions from sequences, until now, considered invariant in all tRNA species. This tRNA is aminoacylated with L-Serine by the seryl-tRNA ligase which also charges cognate tRNASer.
CONCLUSIONS:
Selenocysteine, therefore, is synthesized from a serine residue bound to a natural suppressor tRNA which recognizes UGA.
Proceedings of the National Academy of Sciences,2000,97 (21) 11528-11533.
l-Serine and glycine serve as major astroglia-derived trophic factors for cerebellar Purkinje neurons.[Reference:
WebLink]
Glial cells support the survival and development of central neurons through the supply of trophic factors.
METHODS AND RESULTS:
Here we demonstrate that L-Serine (L-Ser) and glycine (Gly) also are glia-derived trophic factors. These amino acids are released by astroglial cells and promote the survival, dendritogenesis, and electrophysiological development of cultured cerebellar Purkinje neurons. Although L-Ser and Gly are generally classified as nonessential amino acids, 3-phosphoglycerate dehydrogenase (3PGDH), a key enzyme for their biosynthesis, is not expressed in Purkinje neurons. By contrast, the Bergman glia, a native astroglia in the cerebellar cortex, highly expresses 3PGDH.
CONCLUSIONS:
These data suggest that L-Ser and Gly mediate the trophic actions of glial cells on Purkinje neurons.
Microbiology, 2003, 149(8):2023-2030.
L-Serine, D- and L-proline and alanine as respiratory substrates of Helicobacter pylori: correlation between in vitro and in vivo amino acid levels.[Reference:
WebLink]
METHODS AND RESULTS:
Helicobacter pylori whole cells showed high rates of oxygen uptake with L-Serine and L-proline as respiratory substrates, and somewhat lower rates with D-alanine and D-proline. These respiratory activities were inhibited by rotenone and antimycin A at low concentrations. Since pyruvate was produced from L-Serine and D- and L-alanine in whole cells, the respiratory activities with these amino acids as substrates occurred via pyruvate. Whole cells showed 2,6-dichlorophenolindophenol (DCIP)-reducing activities with D- and L-proline and D-alanine as substrates, suggesting that hydrogen removed from these amino acids also participated in oxygen uptake by the whole cells.
CONCLUSIONS:
High amounts of L-proline, D- and L-alanine, and L-Serine were present in H. pylori cells, and these amino acids also predominated in samples of human gastric juice. H. pylori seems to utilize D- and L-proline, D-alanine and L-Serine as important energy sources in its habitat of the mucous layer of the stomach.
Prog Neuropsychopharmacol Biol Psychiatry, 2008, 32(8):1905-1912.
Changes in plasma glycine, L-serine, and D-serine levels in patients with schizophrenia as their clinical symptoms improve: results from the Juntendo University Schizophrenia Projects (JUSP).[Reference:
WebLink]
Based on the hypothesis of NMDA receptor hypofunction in schizophrenia, plasma glycine, L-Serine, and D-serine levels have been studied, since they could serve as biological markers. However, changes over time in the levels of these amino acids in schizophrenic patients have not been investigated.
To clarify the mean plasma glycine, L-Serine, and D-serine levels in patients with schizophrenia, levels of these amino acids were compared between healthy controls and patients with schizophrenia. The plasma levels of these amino acids during the clinical course of schizophrenia were also compared.
METHODS AND RESULTS:
Eighty-nine Japanese patients with schizophrenia and 50 age- and gender-matched healthy controls were studied. Plasma glycine, L-Serine, and D-serine levels and their ratios were measured twice, during the acute stage and during the remission stage, using high-performance liquid chromatography. The admission plasma glycine, L-Serine, and D-serine levels of schizophrenic patients were higher than those of healthy controls. There were no significant differences between drug-naïve patients and healthy controls in the admission levels of the plasma amino acids, but chronically medicated patients had higher admission plasma glycine and D-serine levels. Only the D-serine level and the D-/L-Serine ratio were markedly significantly increased in schizophrenic patients from the time of admission to the time of discharge as their clinical symptoms improved. In addition, the increase in the plasma D-serine levels of drug-naïve patients was correlated with improvements in positive symptoms.
CONCLUSIONS:
Plasma amino acid levels, especially D-serine levels, could be useful as a "therapeutic" or "clinical state" marker in patients with acute schizophrenia.