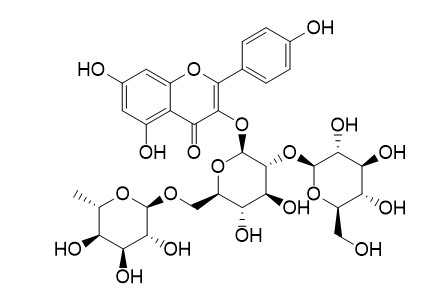
Kaempferol-3-O-(2''-O-beta-D-glucopyl)-beta-D-rutinoside
Kaempferol-3-O-(2''-O-β-D-glucopyl)-β-D-rutinoside is a natural glycoside that could be found in Camellia oleifera seeds.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Polytechnic University of Catalonia2017, 105826
Pharmaceuticals (Basel).2024, 17(4):462.
Korean Journal of Plant Resources2021, 34(1):52-58.
Int J Mol Med.2015, 35(5):1237-45
LWT2020, 110397
Molecules.2021, 26(13):4081.
Phytomedicine.2024, 122:155065.
Toxicol In Vitro.2018, 52:94-105
Indian J. of Experimental Bio.2020, 9(58).
J of Essential Oil Research2019, 1677272
Related and Featured Products
Volume 67, Issue 1, 18 May 2009, Pages 31-37
Extraction and purification of flavanone glycosides and kaemferol glycosides from defatted Camellia oleifera seeds by salting-out using hydrophilic isopropanol[Reference:
WebLink]
The purpose of this research was to investigate a salting-out procedure for isolating four flavonoid glycosides from defatted Camellia oleifera seeds. The procedure included extraction with 80% methanol, methanol removal and addition of an equal amount of hydrophilic isopropanol and salt to separate the isopropanol fraction from the water layer. Using successive column chromatography, kaemferol-3-O-[2-O-β-d-glucopyranosyl-6-O-α-l-rhamnopyranosyl]-β-d-glucopyranoside (compound 1), kaemferol-3-O-[2-O-β-d-xylopyranosyl-6-O-α-l-rhamnopyranosyl]-β-d-glucopyranoside (compound 2), naringenin-7-O-[β-d-xylopyranosyl(1 → 6)][β-d-glucopyranosyl(1 → 3)-α-l-rhamnopyranosyl(1 → 2)]-β-d-glucopyranoside (compound 3) and naringenin-7-O-β-d-xylopyranosyl(1 → 6)-β-d-glucopyranoside (compound 4) were obtained. The structure of compound 3, a new flavanone glycoside, was analyzed using UV, FT-IR, 1H NMR, 13C NMR and HR-FAB-MS. Quantification using high-performance liquid chromatography (HPLC) demonstrated that defatted C. oleifera seed cake contains 7.92, 17.7, 2.23 and 1.06 mg/g of the four compounds. The extraction efficiencies of the four compounds were increased by 23.17%, 7.59%, 48.67% and 47.22% from those obtained with n-butanol partition extraction. This new extraction technique is simpler, faster and less expensive than the traditional extraction method. Accordingly, this salting-out method can potentially replace the existing one.