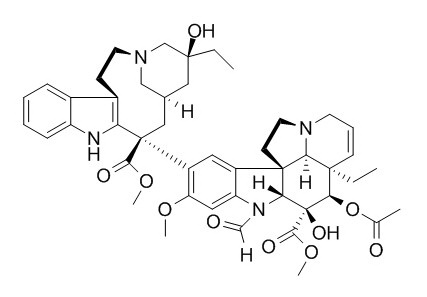
Vincristine
Vincristine-induced nociceptive painful sensation, may be due to its potential of antioxidative, neuroprotective and calcium channel inhibitory action.Vincristine can treat MM, ERK1/2, Akt, and NF-κB inhibitors are potentially useful as anti-MDR agents for the treatment of Vincristine-resistant MM. An inherited polymorphism in the promoter region of CEP72 was associated with increased risk and severity of Vincristine-related peripheral neuropathy.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
JEJU National University2022, 24032.
Food Res Int.2018, 106:909-919
Horticulturae2021, 7(1),5.
Horticulturae2023, 9(2), 213.
South African J of Botany2020, 135:50-57
Evid Based Complement Alternat Med.2017, 2017:1583185
Int J Biol Macromol.2025, 292:139225.
Front Plant Sci.2023, 14:1207940.
J Food Biochem.2020, 44(6):e13198.
Chemistry of Vegetable Raw Materials2019, 3:119-127
Related and Featured Products
Clin J Oncol Nurs . 2018 Dec 1;22(6):669-672.
Vincristine Minibag Administration: A Quality Improvement Project to Minimize Medical Errors[Pubmed:
30452001]
Abstract
Vincristine is a cytotoxic chemotherapy agent classified as an antitumor alkaloid and is part of the vinca alkaloid family. Vincristine's mechanism of action is to primarily inhibit mitosis of the cancer cell and is given by IV route only for treatment. Accidental intrathecal administration of Vincristine has lethal consequences for patients. To minimize the risk of accidental intrathecal administration of Vincristine, 14 infusion centers participated in a quality improvement project to change the practice of Vincristine administration from IV push to IV piggyback via minibag and gravity. After three months, all infusion centers successfully implemented the practice.
Keywords: nurse skill training; patient safety; Vincristine IV piggyback administration.
Toxicol Ind Health. 2014 Oct;30(9):794-805.
Ameliorative effect of Vernonia cinerea in vincristine-induced painful neuropathy in rats.[Pubmed:
23081859]
The present study was designed to investigate the antinociceptive potential of Vernonia cinerea (VC) on Vincristine-induced painful neuropathy in rats.
METHODS AND RESULTS:
A chemotherapeutic agent, Vincristine (50 μg/kg intraperitoneally for 10 consecutive days), was administered for the induction of neuropathic pain in rats. The painful behavioral changes were assessed using hot plate, acetone drop, paw pressure, Von Frey hair and tail immersion tests to assess the degree of hyperalgesic and allodynic pain sensation in paw and tail. Tissue biomarker changes including thiobarbituric acid reactive substances (TBARSs), reduced glutathione (GSH) and total calcium levels were estimated in sciatic nerve tissue samples to assess the degree of oxidative stress. Histopathological changes were also observed in transverse sections of rat sciatic nerve tissue. Ethanolic extract of VC leaves and pregabalin were administered for 14 consecutive days from day 0 (day of surgery). Pregabalin served as a positive control in the present study. Vincristine administration resulted in a significant reduction in painful behavioral changes along with a rise in the levels of TBARS, total calcium and decrease in GSH levels when compared with the normal control group. Furthermore, significant histopathological changes were also observed.
CONCLUSIONS:
Pretreatment with VC significantly attenuated Vincristine-induced development of painful behavioral, biochemical and histological changes in a dose-dependent manner, which is similar to that of pregabalin-pretreated group. The attenuating effect of VC in Vincristine-induced nociceptive painful sensation may be due to its potential of antioxidative, neuroprotective and calcium channel inhibitory action.
JAMA. 2015 Feb 24;313(8):815-23.
Association of an inherited genetic variant with vincristine-related peripheral neuropathy in children with acute lymphoblastic leukemia.[Pubmed:
25710658]
To identify genetic germline variants associated with the occurrence or severity of Vincristine-induced peripheral neuropathy in children with ALL.
METHODS AND RESULTS:
Genome-wide association study of patients in 1 of 2 prospective clinical trials for childhood ALL that included treatment with 36 to 39 doses of Vincristine. Genome-wide single-nucleotide polymorphism (SNP) analysis and Vincristine-induced peripheral neuropathy were assessed in 321 patients from whom DNA was available: 222 patients (median age, 6.0 years; range, 0.1-18.8 years) enrolled in 1994-1998 in the St Jude Children's Research Hospital protocol Total XIIIB with toxic effects follow-up through January 2001, and 99 patients (median age, 11.4 years; range, 3.0-23.8 years) enrolled in 2007-2010 in the Children's Oncology Group (COG) protocol AALL0433 with toxic effects follow-up through May 2011. Human leukemia cells and induced pluripotent stem cell neurons were used to assess the effects of lower CEP72 expression on Vincristine sensitivity. Treatment with Vincristine at a dose of 1.5 or 2.0 mg/m2. Vincristine-induced peripheral neuropathy was assessed at clinic visits using National Cancer Institute criteria and prospectively graded as mild (grade 1), moderate (grade 2), serious/disabling (grade 3), or life threatening (grade 4). Grade 2 to 4 Vincristine-induced neuropathy during continuation therapy occurred in 28.8% of patients (64/222) in the St Jude cohort and in 22.2% (22/99) in the COG cohort. A SNP in the promoter region of the CEP72 gene, which encodes a centrosomal protein involved in microtubule formation, had a significant association with Vincristine neuropathy (meta-analysis P = 6.3×10(-9)). Reducing CEP72 expression in human neurons and leukemia cells increased their sensitivity to Vincristine.
CONCLUSIONS:
In this preliminary study of children with ALL, an inherited polymorphism in the promoter region of CEP72 was associated with increased risk and severity of Vincristine-related peripheral neuropathy. If replicated in additional populations, this finding may provide a basis for safer dosing of this widely prescribed anticancer agent.
Leuk Res. 2015 Apr;39(4):445-52.
Overexpression of survivin via activation of ERK1/2, Akt, and NF-κB plays a central role in vincristine resistance in multiple myeloma cells.[Pubmed:
25726084]
The acquisition of anti-cancer drug resistance is a major limitation of chemotherapy for multiple myeloma (MM) and it is thus important to identify the mechanisms by which MM cells develop such drug resistance. In a previous study, we showed that multidrug resistance (MDR) involves the overexpression of MDR1 and survivin in Vincristine-resistant RPMI8226/VCR cells. However, the underlying mechanism of MDR remains unclear.
METHODS AND RESULTS:
In this study, we investigated the mechanism of MDR in RPMI8226/VCR cells, and found that RPMI8226/VCR cells exhibit increased levels of activated ERK1/2, Akt, and NF-κB, while the levels of activated mTOR, p38MAPK, and JNK do not differ between RPMI8226/VCR cells and their Vincristine-susceptible counterparts. In addition, the inhibition of ERK1/2, Akt, or NF-κB by inhibitors reversed the drug-resistance of RPMI8226/VCR cells via the suppression of survivin expression, but did not affect MDR1 expression; RNA silencing of survivin expression completely reversed Vincristine resistance, while MDR1 silencing only weakly suppressed Vincristine resistance in RPMI8226/VCR cells.
CONCLUSIONS:
These results indicate that enhanced survivin expression via the activation of ERK1/2, Akt, and NF-κB plays a critical role in Vincristine resistance in RPMI8226/VCR cells. Our findings suggest that ERK1/2, Akt, and NF-κB inhibitors are potentially useful as anti-MDR agents for the treatment of Vincristine-resistant MM.