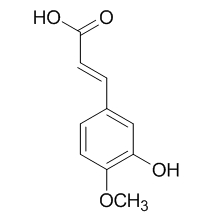
Isoferulic acid
Isoferulic acid is an effective natural antioxidant in both lipid and aqueous media, it may be a new promising anti-glycation agent for the prevention of diabetic complications via inhibition of advanced glycation end products (AGEs) formation and oxidation-dependent protein damage. Isoferulic acid is a novel and potent inhibitor of murine IL-8 production, it also has inhibitory effect on mushroom tyrosinase.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Primary and Industrial.2018, 52(11)
Environ Toxicol.2020, doi: 10.1002
Applied Biological Chemistry2023, 66(58):112.
Oxid Med Cell Longev.2021, 2021:6647107.
PLoS One.2018, 13(3):e0193386
J Adv Res.2021, 35:245-257.
J Sci Food Agric.2024, 104(7):4425-4437.
J Nat Prod.2023, 86(2):264-275.
Viruses.2024, 16(7):1128.
J Nat Med.2020, 74(3):550-560.
Related and Featured Products
Molecules. 2013 May 30;18(6):6439-54.
Isoferulic acid, a new anti-glycation agent, inhibits fructose- and glucose-mediated protein glycation in vitro.[Pubmed:
23722732]
The inhibitory activity of Isoferulic acid (IFA) on fructose- and glucose-mediated protein glycation and oxidation of bovine serum albumin (BSA) was investigated.
METHODS AND RESULTS:
Our data showed that IFA (1.25-5 mM) inhibited the formation of fluorescent advanced glycation end products (AGEs) and non-fluorescent AGE [Nε-(carboxymethyl) lysine: CML], as well as the level of fructosamine. IFA also prevented protein oxidation of BSA indicated by decreasing protein carbonyl formation and protein thiol modification. Furthermore, IFA suppressed the formation of β-cross amyloid structures of BSA.
CONCLUSIONS:
Therefore, IFA might be a new promising anti-glycation agent for the prevention of diabetic complications via inhibition of AGEs formation and oxidation-dependent protein damage.
Nat Prod Commun. 2011 Sep;6(9):1285-8.
Evaluation of antioxidant activity of isoferulic acid in vitro.[Pubmed:
21941899]
Isoferulic acid (3-hydroxy-4-methoxycinnamic acid, IFA), the isomer of ferulic acid (4-hydroxy-3-methoxycinnamic acid), is a rare phenolic acid occurring in Rhizoma Cimicifugae. Unlike ferulic acid, which has been well investigated, the antioxidant activity of IFA has not been measured.
METHODS AND RESULTS:
In this study, IFA was systematically evaluated for its in vitro antioxidant activity for the first time. IC50 values were calculated of 7.30 +/- 0.57, 4.58 +/- 0.17, 1.08 +/- 0.01, 8.84 +/- 0.43, 7.69 +/- 0.39, 1.57 +/- 0.2, 13.33 +/- 0.49 microg/mL, respectively, for lipid peroxidation, DPPH (1,1-diphenyl-2-picrylhydrazyl radical) and ABTS (3-ethylbenzthiazoline-6-sulfonic acid diammonium salt) radical scavenging, reducing power on Fe3+ and CU2+ ions, and hydroxyl and superoxide anion radical scavenging. Comparison with the IC50 values with those of the positive controls, Trolox and butylated hydroxyanisole (BHA), it can be concluded that Isoferulic acid is an effective natural antioxidant in both lipid and aqueous media.
Planta Med. 1995 Jun;61(3):221-6.
Inhibitory effect of ferulic acid and isoferulic acid on murine interleukin-8 production in response to influenza virus infections in vitro and in vivo.[Pubmed:
7617763 ]
We investigated the effect of ferulic acid (FA) and Isoferulic acid (IFA), which are active components of the rhizoma of Cimicifuga species used frequently as anti-inflammatory drugs in Japanese Oriental medicines, on murine interleukin-8 (IL-8) production in response to influenza virus infections in vitro and in vivo by antibody-sandwich enzyme-linked immunosorbent assay.
METHODS AND RESULTS:
In the in vitro study, the murine macrophage cell line RAW 264.7 was infected with influenza virus at a dose of 10 plaque forming units (PFU)/cell and cultured in the presence or absence of drugs. Both FA and IFA reduced the IL-8 levels in the 20-h conditioned medium in comparison with control in a dose-dependent manner. The effect of IFA was greater than that of FA: IL-8 levels were reduced to 43% and 56% of the control in the presence of 100 micrograms/ml of IFA and FA, respectively. In the in vivo study, mice were infected with 1,000 PFU of virus and received daily oral administrations of Cimicifuga heracleifolia extract (5 mg/mouse/day), FA (0.5 mg/mouse/day), IFA (0.125 mg/mouse/day), or phosphate buffered saline. The three drugs showed a tendency to reduce IL-8 levels in bronchoalveolar lavage (BAL) obtained 2 days after infection. Moreover, both FA and IFA also significantly reduced the number of exuded neutrophils into BAL. However, the drug administrations did not affect the virus yields in BAL.
CONCLUSIONS:
These data suggest that FA and IFA are novel and potent inhibitors of murine IL-8 production and might act as one of the main components of anti-inflammatory rhizoma of Cimicifuga species.
J Cosmet Sci. 2013 Jul-Aug;64(4):235-41.
Kinetics of inhibitory effect of isoferulic acid on mushroom tyrosinase.[Pubmed:
23931087]
A study on the kinetics of inhibitory effect of Isoferulic acid on the monophenolase and diphenolase activity of mushroom tyrosinase was carried out using enzymological kinetic analysis method in a Na2HPO4-NaH2PO4 buffer solution (pH = 6.8) at 30°C.
METHODS AND RESULTS:
It was found that Isoferulic acid efficiently inhibits both monophenolase and diphenolase activities of mushroom tyrosinase under experimental conditions. Concentrations of Isoferulic acid leading to 50% rate inhibition (IC50) on monophenolase and diphenolase activity were calculated to be 0.13 mmol/L and 0.39 mmol/L, respectively, which are much lower than that of arbutin (IC50 = 5.3 mmol/L for diphenolase activity). The presence of Isoferulic acid also prolongs the lag period in the oxidation process of l-tyrosine via tyrosinase-a 4.3-min lagging was observed with the presence of 0.20 mmol/L Isoferulic acid-compared to a 1.1-min lagging in the absence of Isoferulic acid. The Lineweaver-Burk plot demonstrates a competitive behavior of Isoferulic acid in the tyrosinase oxidation of l-3,4-dihydroxyphenylalanine, with maximum reaction rate (vm) and inhibition constant (KI) at 64.5 µM/min and 0.11 mmol/L, respectively.
Bioorg Med Chem. 2014 May 1;22(9):2707-13.
Search for novel histone deacetylase inhibitors. Part II: design and synthesis of novel isoferulic acid derivatives.[Pubmed:
24702857]
Previously, we described the discovery of potent ferulic acid-based histone deacetylase inhibitors (HDACIs) with halogeno-acetanilide as novel surface recognition moiety (SRM).
METHODS AND RESULTS:
In order to improve the affinity and activity of these HDACIs, twenty seven Isoferulic acid derivatives were described herein. The majority of title compounds displayed potent HDAC inhibitory activity. In particular, IF5 and IF6 exhibited significant enzymatic inhibitory activities, with IC50 values of 0.73 ± 0.08 and 0.57 ± 0.16 μM, respectively. Furthermore, these compounds showed moderate antiproliferative activity against human cancer cells. Especially, IF6 displayed promising profile as an antitumor candidate with IC50 value of 3.91 ± 0.97 μM against HeLa cells.
CONCLUSIONS:
The results indicated that these Isoferulic acid derivatives could serve as promising lead compounds for further optimization.
Br J Pharmacol. 2000 Feb;129(4):631-6.
Antihyperglycemic action of isoferulic acid in streptozotocin-induced diabetic rats.[Pubmed:
10683186]
Wistar rats with streptozotocin-induced diabetes (STZ-diabetic rats), which is similar to human insulin-dependent diabetic mellitus (IDDM), were employed to investigate the antihyperglycemic action of Isoferulic acid.
METHODS AND RESULTS:
A single intravenous injection of Isoferulic acid decreased the plasma glucose in a dose-dependent manner in the STZ-diabetic rats. Repeated intravenous administration of STZ-diabetic rats with Isoferulic acid (5.0 mg kg(-1)) also resulted in the lowering of plasma glucose after one day. Stimulatory effects of Isoferulic acid on the glucose uptake and glycogen synthesis in soleus muscles isolated from STZ-diabetic rats were also obtained indicating an increase of glucose utilization following Isoferulic acid treatment which was not dependent on insulin. The mRNA level of glucose transporter subtype 4 form (GLUT4) in soleus muscle was raised by Isoferulic acid after repeated treatment for 1 day in STZ-diabetic rats. Similar repeated treatment with Isoferulic acid reversed the elevated mRNA level of phosphoenolpyruvate carboxykinase (PEPCK) in liver of STZ-diabetic rats to the normal level. However, expression of GLUT4 and PEPCK genes in nondiabetic rats were not influenced by similar treatment with Isoferulic acid.
CONCLUSIONS:
These results suggest that Isoferulic acid can inhibit hepatic gluconeogenesis and/or increase the glucose utilization in peripheral tissue to lower plasma glucose in diabetic rats lacking insulin.