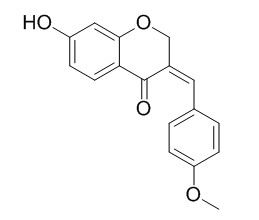
Isobonducellin
Isobonducellin exhibits potent cytotoxic activity in Jurkat and HepG2 cells, while moderate growth inhibition against Colon205 cells.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
J Pharm Pharmacol.2024, 76(10):1239-1268.
PLoS One.2021, 16(6):e0248479.
BMC Complement Altern Med.2016, 16:213
Nutrients.2018, 10(7)
J Pharm Biomed Anal.2024, 241:115990.
Toxicol In Vitro.2019, 59:161-178
Food Bioscience2023, 56:103311.
Sci Adv.2018, 4(10)
Molecules2022, 27(11):3606.
J Cell Mol Med.2023, jcmm.18071.
Related and Featured Products
Phytochemistry. 2004 Sep;65(17):2455-61.
Stimulating the production of homoisoflavonoids in cell suspension cultures of Caesalpinia pulcherrima using cork tissue.[Pubmed:
15381409]
It has previously been demonstrated that cork tissue increases the efficiency of the production of lipophilic secondary metabolites in diverse plant cell suspension cultures.
METHODS AND RESULTS:
In the present study, three new homoisoflavonoids--named dihydrobonducellin, 2'-methoxydihydrobonducellin, and 2'-methoxybonducellin--and bonducellin and Isobonducellin were isolated from Caesalpinia pulcherrima cultured cells coincubated with cork tissue. Cork tissue increased the production of 2'-methoxybonducellin by about 7-fold relative to control cells, and more than 80% of the product was recoverable from the cork tissue. When cork tissue and methyl jasmonate or yeast extract were added simultaneously to the medium, the amount of 2'-methoxybonducellin produced increased further. The production of the other four homoisoflavonoids was enhanced by variable amounts.
CONCLUSIONS:
Our results indicate that the addition of cork tissue would be an effective technique for investigating formation of secondary metabolites that usually accumulate only in trace amounts.
Food Chem Toxicol. 2007 Sep;45(9):1770-6.
Antioxidant and cytotoxic activities of naturally occurring phenolic and related compounds: a comparative study.[Pubmed:
17475387]
The antioxidant (DPPH radical and superoxide anion scavenging activities), and cytotoxic (in tumor, Jurkat, PC-3, Colon 205, HepG2, and normal PBMCs cells) activities of 16 plant phenolic or related compounds were evaluated in vitro.
METHODS AND RESULTS:
Different categories compounds corresponding to 10 flavonoids, three lignans, two phenolic acids, and a catechin showed significant mean differences in antioxidant and cytotoxic activities. Particularly, the flavonols, quercetin (3) and tiliroside (11) possess significant antioxidant activity, as well as cytotoxic activity against Jurkat; and Jurkat and HepG2 cells, respectively. In contrast, the flavanone, 5,7-dimethoxy-3',4'-methylenedioxyflavanone (7), and homoisoflavonoid, Isobonducellin (10) shown to have no significant antioxidant activity, but exhibited potent cytotoxic activity in Jurkat and HepG2 cells, while moderate growth inhibition against Colon205 cells. Interestingly, none of these derivatives shown to have toxicity toward normal peripheral blood mononuclear cells, over the concentration range tested (5-200 microM).
CONCLUSIONS:
Cytotoxic activities of some natural flavonoids identified in the medicinal plants were evaluated for the first time.
Phytochemistry. 2003 Aug;63(7):789-93.
Flavanoids from Caesalpinia pulcherrima.[Pubmed:
12877920 ]
METHODS AND RESULTS:
Two new flavanoids, 5,7-dimethoxy-3',4'-methylenedioxyflavanone and Isobonducellin along with 2'-hydroxy-2,3,4',6'-tetramethoxychalcone, 5,7-dimethoxyflavone and bonducellin were isolated from the aerial parts of Caesalpinia pulcherrima. The structures of the compounds were settled mainly by interpretation of their 1D and 2D NMR spectra.
CONCLUSIONS:
Isobonducellin was found to be a homoisoflavanoid containing a cis (Z)-double bond. Antimicrobial activity of the new compounds was evaluated.