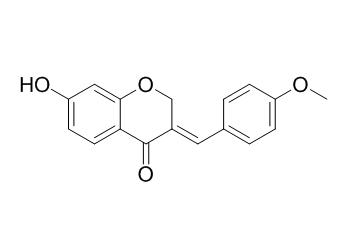
Bonducellin
Bonducellin has anti-inflammatory activities, it can significantly and dose-dependently inhibit the inflammatory mediators; nitric oxide (NO), and cytokines [tumor necrosis factor (TNF)-alpha and interleukin (IL)-12. Bonducellin shows weak antimalarial activity against Plasmodium falciparum with the IC50 value of 26 uM; it also shows weak antiproliferative against A2780 human ovarian cancer cell line , with the IC50 value of 10.6 uM. Bonducellin shows modulation in the MIC of EtBr by eight fold at a concentration of 62.5 mg/L and also shows significant efflux pump inhibitory activity.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Korean J of Medicinal Crop Science2018, 220-226
Eur J Neurosci.2021, 53(11):3548-3560.
Int J Mol Sci.2024, 25(17):9730.
Biomolecules.2022, 12(12):1754.
Chinese Journal of Tissue Engineering Research2024, 28(8):1149-1154.
Research on Crops.2017, 18(2)
J Nat Med.2017, 71(2):457-462
Acta horticulturae2017, 1158:257-268
Int J Mol Sci.2024, 25(12):6456.
Appl. Sci. 2021, 11(23),11099.
Related and Featured Products
Bioorg Med Chem. 2013 Dec 15;21(24):7591-4.
Bioactive compounds from Stuhlmannia moavi from the Madagascar dry forest.[Pubmed:
24239390 ]
METHODS AND RESULTS:
Bioassay-directed fractionation of the leaf and root extracts of the antiproliferative Madagascar plant Stuhlmannia moavi afforded 6-acetyl-5,8-dihydroxy-2-methoxy-7-methyl-1,4-naphthoquinone (stuhlmoavin, 1) as the most active compound, with an IC50 value of 8.1 μM against the A2780 human ovarian cancer cell line, as well as the known homoisoflavonoid Bonducellin (2) and the stilbenoids 3,4,5'-trihydroxy-3'-methoxy-trans-stilbene (3), piceatannol (4), resveratrol (5), rhapontigenin (6), and isorhapontigenin (7). The structure elucidation of all compounds was based on NMR and mass spectroscopic data, and the structure of 1 was confirmed by a single crystal X-ray analysis.
CONCLUSIONS:
Compounds 2-5 showed weak A2780 activities, with IC50 values of 10.6, 54.0, 41.0, and 74.0 μM, respectively. Compounds 1-3 also showed weak antimalarial activity against Plasmodium falciparum with IC50 values of 23, 26, and 27 μM, respectively.
Eur J Med Chem. 2013 Aug;66:499-507.
7-Hydroxy-(E)-3-phenylmethylene-chroman-4-one analogues as efflux pump inhibitors against Mycobacterium smegmatis mc2 155.[Pubmed:
23832254]
Efflux pump (EP) induces resistance in mycobacteria and hence could be explored as a new target for the discovery of anti-TB agents. In search for efflux pump inhibitors from natural products, Bonducellin, a homoisoflavonoid was isolated from Caesalpinia digyna roots and evaluated for modulation and EP inhibitory activity.
METHODS AND RESULTS:
Bonducellin showed modulation in the MIC of EtBr by eight fold at a concentration of 62.5 mg/L and also showed significant EP inhibitory activity. A synthetic scheme was designed to prepare analogues of 7-hydroxy-(E)-3-phenylmethylene-chroman-4-one by modification at the phenylmethylene-ring and the synthesized compounds were evaluated in accumulation and efflux assays.
CONCLUSIONS:
Analogues 1, 7-11, 13-15, 17 and 19 were found to be good modulators and decreased the MIC of EtBr by ≥4 fold at sub-inhibitory concentration. The compounds 8, 13 and 17 were the most potent inhibitors of ethidium bromide efflux in Mycobacterium smegmatis mc(2) 155.
J Ethnopharmacol. 2005 Sep 14;100(3):249-53.
Anti-inflammatory activities of flavonoids isolated from Caesalpinia pulcherrima.[Pubmed:
15893896]
METHODS AND RESULTS:
The anti-inflammatory activities of five flavonoids, namely 5,7-dimethoxyflavanone (1), 5,7-dimethoxy-3',4'-methylenedioxyflavanone (2), isoBonducellin (3), 2'-hydroxy-2,3,4',6'-tetramethoxychalcone (4) and Bonducellin (5), all of them isolated from Caesalpinia pulcherrima L. was studied in lipopolysaccharide (LPS) and interferon (IFN)-gamma activated murine peritoneal macrophages. These five compounds significantly and dose-dependently inhibited the inflammatory mediators; nitric oxide (NO), and cytokines [tumor necrosis factor (TNF)-alpha and interleukin (IL)-12]. According to their inhibitory results, the order of anti-inflammatory potency was compounds 3>5>4>2>1. Furthermore, peritoneal macrophages were pre-activated with LPS/IFN-gamma for 24h, and determined the inhibitory effects of the above-mentioned isolates on the production of NO after a further 24h.
CONCLUSIONS:
The present study supports the use of Caesalpinia pulcherrima for the treatment of inflammatory diseases in traditional medicine. This is the first study on compounds 1-5 about their anti-inflammatory activities.