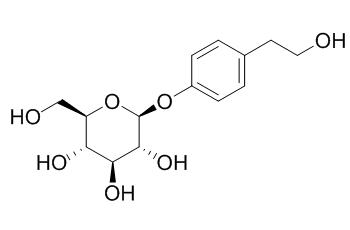
Icariside D2
Icariside D2 shows significant cytotoxic activity on the HL-60 cell line with the IC50 value of 9.0 ± 1.0 uM, it induces apoptosis via alteration of expression of apoptosis-related proteins and decreased phosphorylation of AKT in HL-60 cells.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Oncotarget.2017, 8(64):108006-108019
Phytochem Anal.2016, 27(5):296-303
Biochem Biophys Res Commun.2019, 518(4):732-738
Sci Adv.2018, 4(10)
Nutrients2020, 12(3):811.
Pharmaceuticals (Basel).2024, ;17(8):1018.
Evid Based Complement Alternat Med.2021, 2021:5023536.
Mol Med Rep.2024, 29(2):26.
Biochem Biophys Res Commun.2020, 530(1):4-9.
Antioxidants (Basel).2021, 10(10):1620.
Related and Featured Products
Pharm Biol. 2015;53(11):1602-7
Chemical constituents of the Annona glabra fruit and their cytotoxic activity.[Pubmed:
25856711 ]
METHODS AND RESULTS:
One new, (2E,4E,1'R,3'S,5'R,6'S)-dihydrophaseic acid 1,3'-di-O-β-d-glucopyranoside, and eight known compounds, (2E,4E,1'R,3'S,5'R,6'S)-dihydrophaseic acid 3'-O-β-d-glucopyranoside (2), Icariside D2 (3), Icariside D2 6'-O-β-d-xylopyranoside (4), 3,4-dimethoxyphenyl O-β-d-glucopyranoside (5), 3,4-dihydroxybenzoic acid (6), blumenol A (7), cucumegastigmane I (8), and icariside B1 (9), were isolated from the fruits of A. glabra. Icariside D2 (3) was found to show significant cytotoxic activity on the HL-60 cell line with the IC50 value of 9.0 ± 1.0 μM and did not show cytotoxic activity on the Hel-299 normal cell line. The further test indicated that compound 3 induced apoptosis via alteration of expression of apoptosis-related proteins and decreased phosphorylation of AKT in HL-60 cells.
CONCLUSIONS:
The results suggested that the constituents from A. glabra may contain effective compounds which can be used as anticancer agents.
Food Chem. 2014 Dec 15;165:92-7
Identification of a new angiotensin-converting enzyme (ACE) inhibitor from Thai edible plants.[Pubmed:
25038653]
METHODS AND RESULTS:
Eight Thai edible plants were tested for their inhibitory activity against an angiotensin-converting enzyme (ACE) using an in vitro assay. The methanol extract of Apium graveolens exhibited significant ACE inhibitory activity with an IC50 value of 1.7 mg/ml, and was then subjected to an isolation procedure that resulted in identification of a pure active constituent, junipediol A 8-O-β-d-glucoside (1-β-d-glucosyloxy-2-(3-methoxy-4-hydroxyphenyl)-propane-1,3-diol) (1), which had good ACE inhibitory activity with an IC50 value of 76 μg/ml. Another eight known compounds, isofraxidin-β-d-glucoside (2), roseoside (3), apigenin-7-O-β-d-glucoside (4), luteolin-7-O-β-d-glucoside (5), Icariside D2 (6), apiin (7), chrysoeriol-7-O-β-d-apiosylglucoside (8), and 11,21-dioxo-3 β,15 α,24-trihydroxyurs-12-ene-24-O-β-d-glucopyranoside (9) were also identified.
CONCLUSIONS:
Although each of these five constituents (2-6) isolated from the same fraction as 1 showed no activity at concentrations of 500 μM, together, when each was present at 300 μg/ml, they enhanced the inhibitory activity of 500 μM of 1 from 64% to 81%.