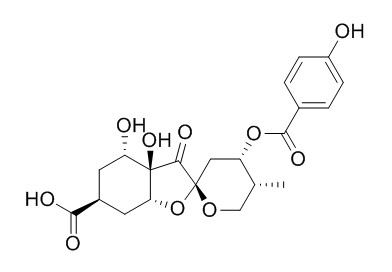
Glochicoccin D
Standard reference
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Clin Transl Med.2021, 11(5):e392.
Phytofrontiers2024, 2690-5442.
Chinese Journal of Hospital Pharmacy2020, 40(7)
J Cachexia Sarcopenia Muscle.2022, 13(6):3149-3162.
Pharmacogn Mag.2015, 11(43):562-6
J Sep Sci.2019, 42(21):3352-3362
Biol Pharm Bull.2020, 43(10):1534-1541.
Molecules.2024, 29(5):1050.
Sci Rep.2017, 7:46299
J Nat Prod.2021, 84(9):2544-2553.
Related and Featured Products
Org Biomol Chem. 2014 Nov 21;12(43):8764-74.
Anti-hepatitis B virus activities and absolute configurations of sesquiterpenoid glycosides from Phyllanthus emblica.[Pubmed:
25268491]
METHODS AND RESULTS:
During the process exploring anti-viral compounds from Phyllanthus species, eight new highly oxygenated bisabolane sesquiterpenoid glycoside phyllaemblicins G1–G8 (1–8) were isolated from Phyllanthus emblica, along with three known compounds, phyllaemblicin F (9), phyllaemblic acid (10) and Glochicoccin D (11). Phyllaemblicin G2 (2), bearing a tricyclo [3.1.1.1] oxygen bridge ring system, is an unusual sesquiterpenoid glycoside, while phyllaemblicins G6–G8 (6–8) are dimeric sesquiterpenoid glycosides with two norbisabolane units connecting through a disaccharide. All the structures were elucidated by the extensive analysis of HRMS and NMR data. The relative configuration of phyllaemblicin G2 was constructed based on heteronuclear coupling constants measurement, and the absolute configurations for all new compounds were established by calculated electronic circular dichroism (ECD) using time dependent density functional theory.
CONCLUSIONS:
The sesquiterpenoid glycoside dimers 6–9 displayed potential anti-hepatitis B virus (HBV) activities, especially for the new compound 6 with IC50 of 8.53 ± 0.97 and 5.68 ± 1.75 μM towards the HBV surface antigen (HBsAg) and HBV excreted antigen (HBeAg) secretion, respectively.
J Org Chem. 2010 Nov 5;75(21):7461-4.
Stereoselective α,α'-annelation reactions of 1,3-dioxan-5-ones.[Pubmed:
20936869 ]
METHODS AND RESULTS:
Pyrrolidine enamines derived from three 1,3-dioxan-5-ones undergo α,α'-annelation reactions with methyl α-(bromomethyl)acrylate to produce bridged 2,4-dioxabicyclo[3.3.1]nonane ring systems with complete stereocontrol. Stereochemical outcomes have been rationalized based on steric and stereoelectronic interactions in intermediate boat-like conformations of the 1,3-dioxane ring and subsequent kinetic protonation to set an axial ester group on the cyclohexanone ring.
CONCLUSIONS:
Base-mediated ester epimerization provides the stereochemical array found in the highly oxygenated cyclohexane ring of phyllaemblic acid and glochicoccin B and Glochicoccin D.